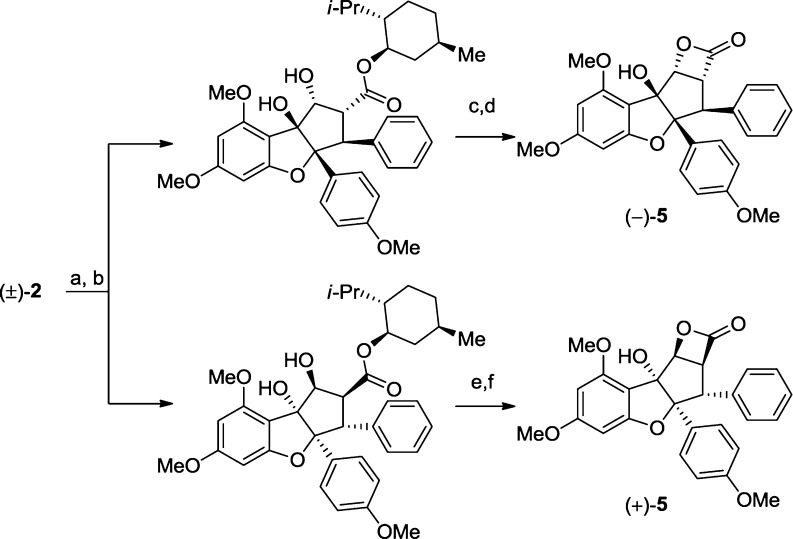
Scheme 4. Synthesis of Both Enantiomers of 5.
Conditions: (a) LiOH, THF, H2O, 60 °C; (b) L-menthol, DCC, DMAP, CH2Cl2, rt (60% combined yield, 2 steps); (c) NaOH, DMSO, H2O, 60 °C, 53% yield; (d) BOP-Cl, Et3N, CH2Cl2, rt, 65% yield; (e) NaOH, DMSO, H2O, 60 °C, 55% yield; (f) BOP-Cl, Et3N, CH2Cl2, rt, 65% yield. DCC = N,N-dicyclohexylcarbodiimide; DMAP = N,N-dimethylaminopyridine; BOP-Cl = bis(2-oxo-3-oxazolidinyl)phosphinic chloride; DMSO = dimethylsulfoxide.