Abstract
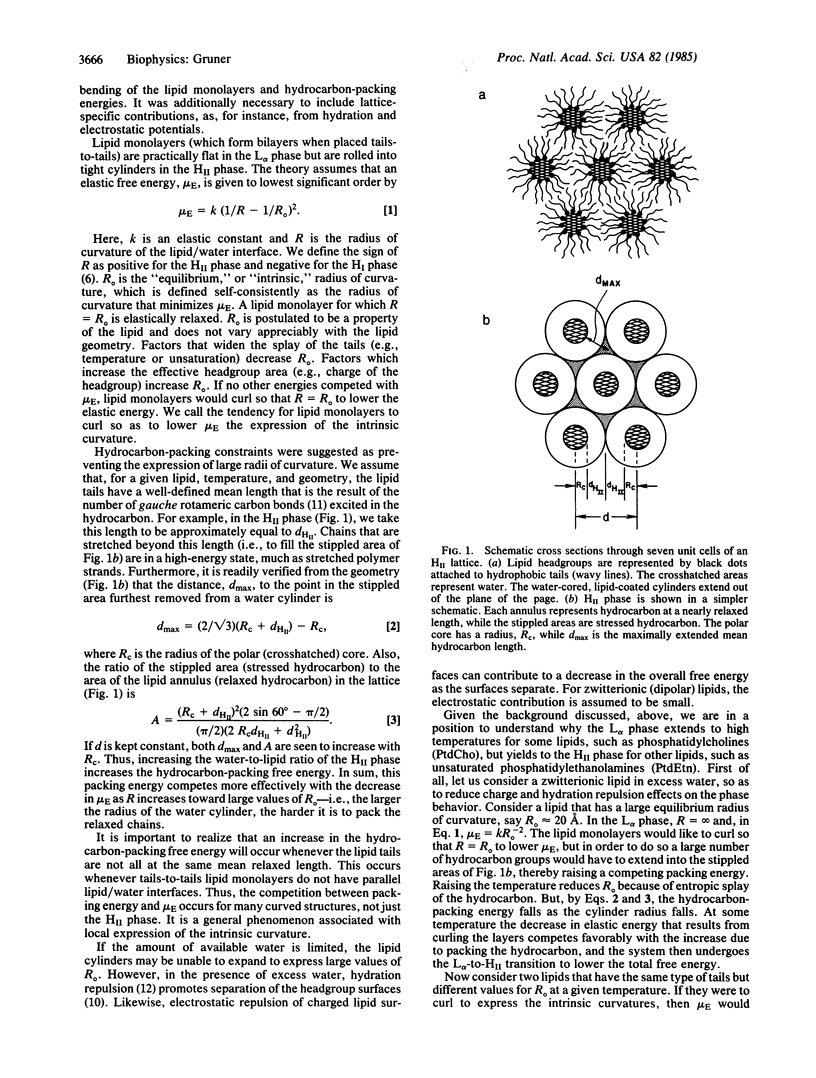
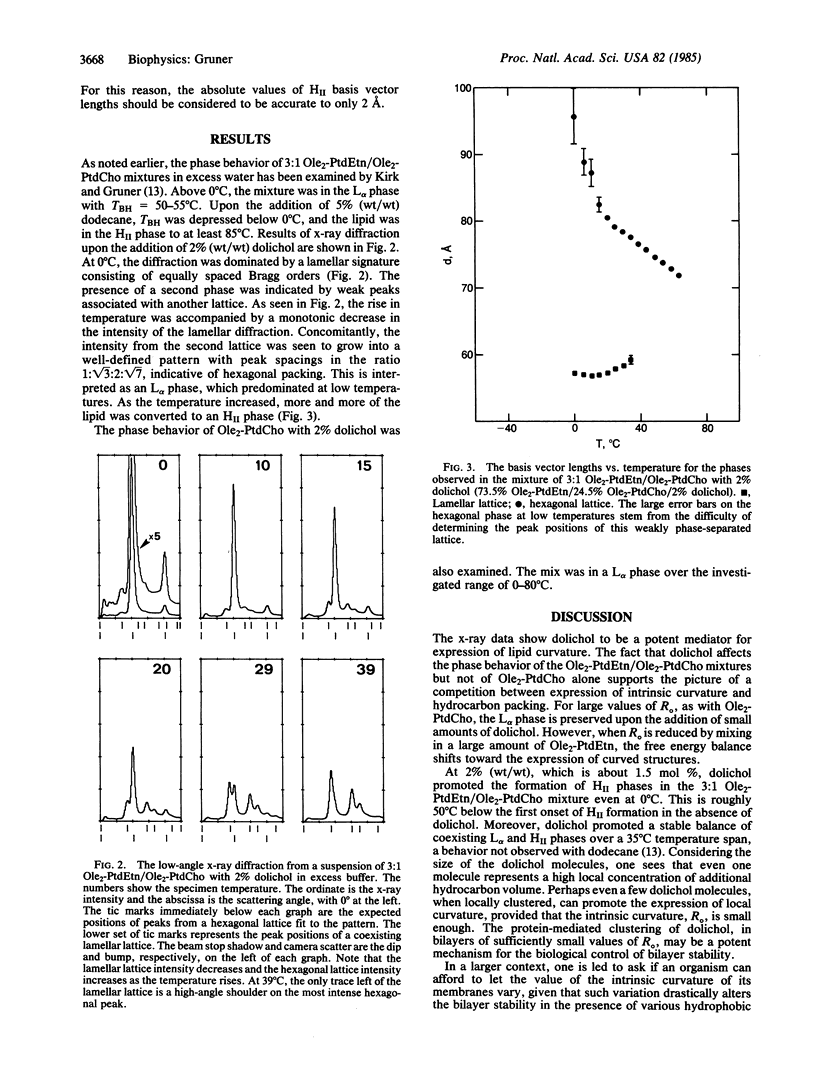
A rationale is presented for the mix of "bilayer" and "nonbilayer" lipids, which occurs in biomembranes. A theory for the L alpha-HII phase transition and experimental tests of the theory are reviewed. It is suggested that the phase behavior is largely the result of a competition between the tendency for certain lipid monolayers to curl and the hydrocarbon packing strains that result. The tendency to curl is quantitatively given by the intrinsic radius of curvature, Ro, which minimizes the bending energy of a lipid monolayer. When bilayer (large Ro) and nonbilayer (small Ro) lipids are properly mixed, the resulting layer has a value of Ro that is at the critical edge of bilayer stability. In this case, bilayers may be destabilized by the protein-mediated introduction of hydrophobic molecules, such as dolichol. An x-ray diffraction investigation of the effect of dolichol on such a lipid mixture is described. This leads to the hypothesis that biomembranes homeostatically adjust their intrinsic curvatures to fall into an optimum range. Experimental strategies for testing the hypothesis are outlined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Haselbeck A., Tanner W. Dolichyl phosphate-mediated mannosyl transfer through liposomal membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1520–1524. doi: 10.1073/pnas.79.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Jensen J. W., Schutzbach J. S. The biosynthesis of oligosaccharide-lipids. Activation of mannosyltransferase II by specific phospholipids. J Biol Chem. 1982 Aug 10;257(15):9025–9029. [PubMed] [Google Scholar]
- Navarro J., Toivio-Kinnucan M., Racker E. Effect of lipid composition on the calcium/adenosine 5'-triphosphate coupling ratio of the Ca2+-ATPase of sarcoplasmic reticulum. Biochemistry. 1984 Jan 3;23(1):130–135. doi: 10.1021/bi00296a021. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Chapman D. The dynamics of membrane structure. CRC Crit Rev Biochem. 1980;8(1):1–117. doi: 10.3109/10409238009105466. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Mak N., McElhaney R. N. Lipid and protein composition and thermotropic lipid phase transitions in fatty acid-homogeneous membranes of Acholeplasma laidlawii B. Biochim Biophys Acta. 1980 Apr 10;597(2):199–215. doi: 10.1016/0005-2736(80)90099-1. [DOI] [PubMed] [Google Scholar]
- Valtersson C., van Duÿn G., Verkleij A. J., Chojnacki T., de Kruijff B., Dallner G. The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem. 1985 Mar 10;260(5):2742–2751. [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]


