Abstract
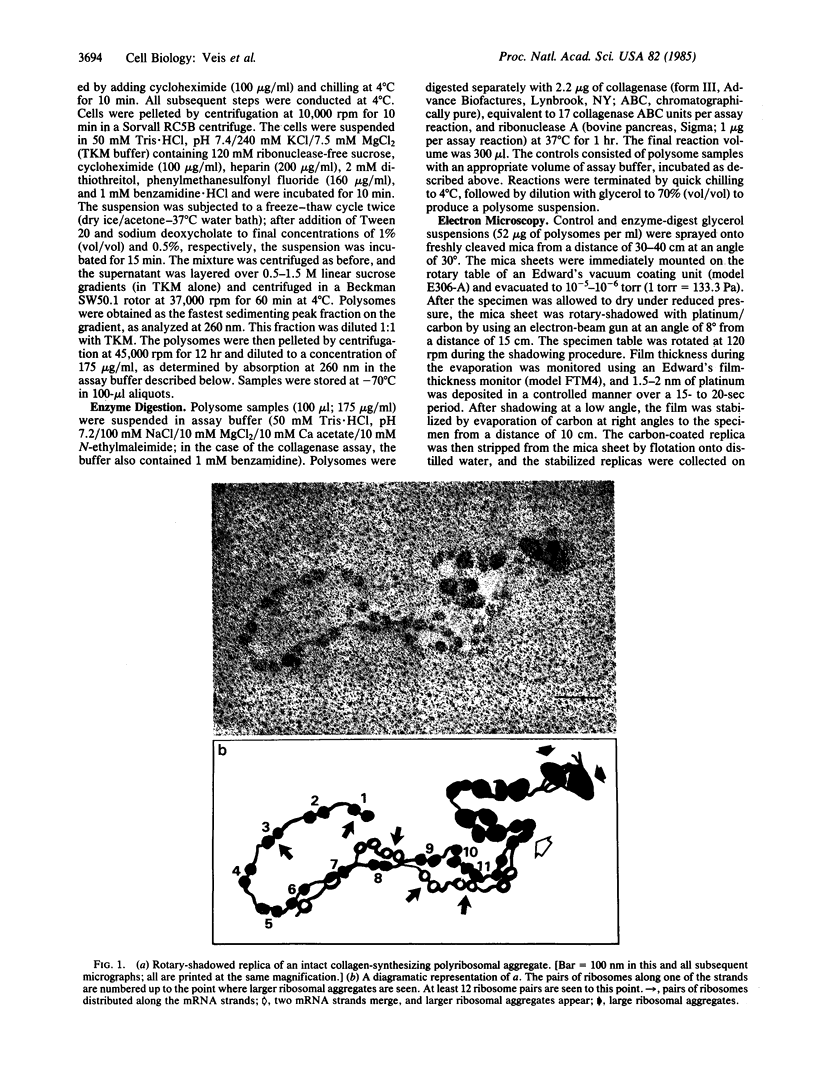
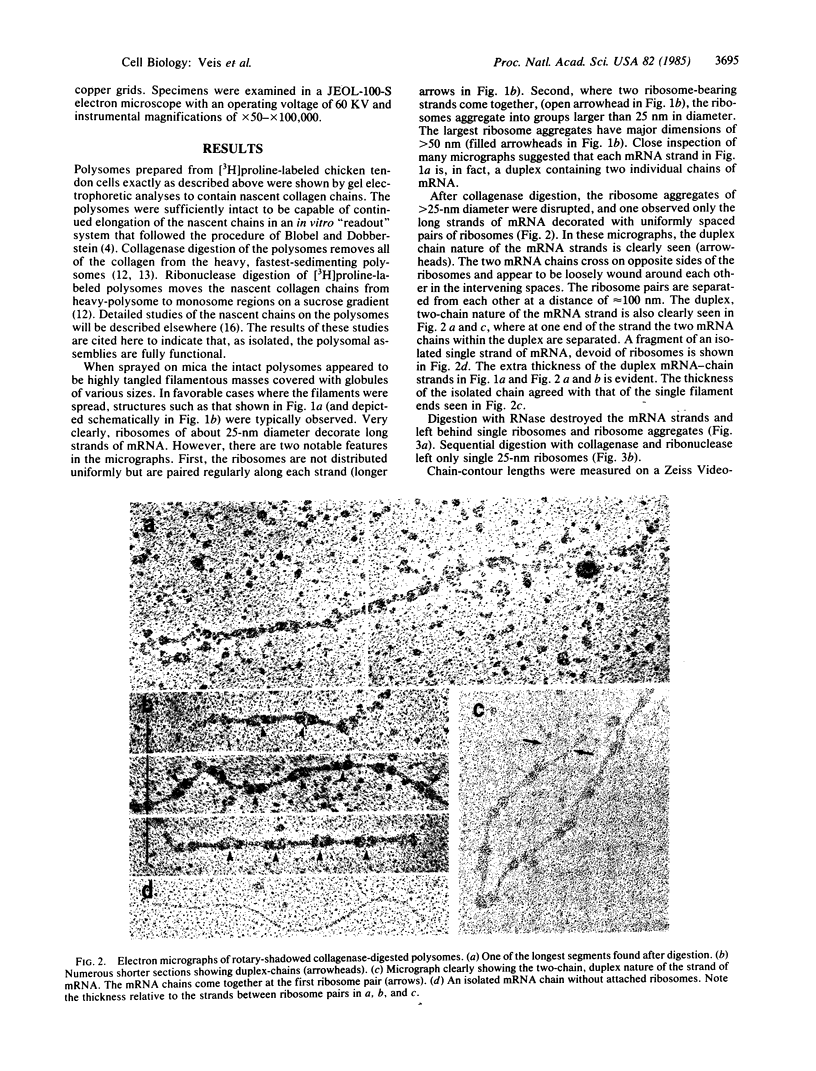
Registration of the three procollagen alpha chains and assembly of the triple-helical procollagen molecules takes place in the rough endoplasmic reticulum, but the exact location and timing of assembly is not known. As part of a study of the mechanism of molecular assembly, intact collagen-producing polyribosomes from embryonic chicken tendon fibroblasts have been examined by the techniques of rotary shadowing and electron microscopy. Intact mRNA strands corresponding in length to approximately 4500 bases and complete procollagen alpha (I) chains have been observed. The mRNA strands are comprised of two mRNA chains. The ribosomes are present in pairs separated along the duplex strand by about 100 nm. The intact polysome is asymmetric; two duplex strands join, and large ribosome aggregates appear. These aggregates are dispersed by collagenase digestion, leaving separate duplex strands with ribosome pairs intact. Ribonuclease digestion yields mixtures of monosomes and ribosome aggregates. Sequential ribonuclease and collagenase digestions yield only monosomes. We propose that each ribosome reads one mRNA chain, so that each pair is thus translating two chains in synchrony. Thus, the complex morphology of the collagen-producing polyribosomes suggests that the organization of a single molecule begins by the organization of the mRNA chains themselves.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Assad R., Peterkofsky B. Characterization of collagen hydroxylysyl glycosyltransferases as mainly intramembranous microsomal enzymes. J Biol Chem. 1984 Jan 25;259(2):854–859. [PubMed] [Google Scholar]
- Brownell A. G., Veis A. Intracellular location of triple helix formation of collagen. Enzyme probe studies. J Biol Chem. 1976 Nov 25;251(22):7137–7143. [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F. Formation of the triple helix of type I procollagen in cellulo. Temperature-dependent kinetics support a model based on cis in equilibrium trans isomerization of peptide bonds. Eur J Biochem. 1984 Apr 16;140(2):391–395. doi: 10.1111/j.1432-1033.1984.tb08114.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F., Prockop D. J. Formation of the triple helix of type I procollagen in cellulo. A kinetic model based on cis-trans isomerization of peptide bonds. Eur J Biochem. 1981 Sep 1;118(3):607–613. doi: 10.1111/j.1432-1033.1981.tb05562.x. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Miller O. L., Jr Electronmicroscopy of genetic activity. Annu Rev Biochem. 1973;42:379–396. doi: 10.1146/annurev.bi.42.070173.002115. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Davidson J. M., Gagnon J., Rowe D. W., Bornstein P. NH2-terminal sequence of the chick proalpha1(I) chain synthesized in the reticulocyte lysate system. Evidence for a transient hydrophobic leader sequence. J Biol Chem. 1979 Mar 10;254(5):1433–1436. [PubMed] [Google Scholar]
- ROSS R., BENDITT E. P. WOUND HEALING AND COLLAGEN FORMATION. IV. DISTORTION OF RIBOSOMAL PATTERNS OF FIBROBLASTS IN SCURVY. J Cell Biol. 1964 Aug;22:365–389. doi: 10.1083/jcb.22.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- Sandell L., Veis A. The molecular weight of the cell-free translation product of alpha l (I) procollagen mRNA. Biochem Biophys Res Commun. 1980 Jan 29;92(2):554–562. doi: 10.1016/0006-291x(80)90369-1. [DOI] [PubMed] [Google Scholar]
- Veis A., Brownell A. G. Collagen biosynthesis. CRC Crit Rev Biochem. 1975 Feb;2(4):417–453. doi: 10.3109/10409237509102549. [DOI] [PubMed] [Google Scholar]
- Veis A., Brownell A. G. Triple-helix formation on ribosome-bound nascent chains of procollagen: deuterium-hydrogen exchange studies. Proc Natl Acad Sci U S A. 1977 Mar;74(3):902–905. doi: 10.1073/pnas.74.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuust J., Piez K. A. A kinetic study of collagen biosynthesis. J Biol Chem. 1972 Feb 10;247(3):856–862. [PubMed] [Google Scholar]
- van Agthoven A., Goridis C., Naquet P., Pierres A., Pierres M. Structural characteristics of the mouse transferrin receptor. Eur J Biochem. 1984 Apr 16;140(2):433–440. doi: 10.1111/j.1432-1033.1984.tb08121.x. [DOI] [PubMed] [Google Scholar]