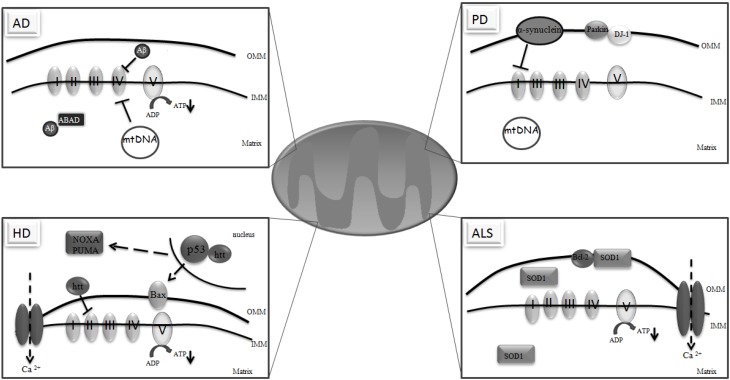
Figure 1.
Mitochondrial dysfunction in neurodegeneration. In Alzheimer´s disease (AD), Aβ accumulates in mitochondria and binds to Aβ-binding alcohol dehydrogenase (ABAD) inhibiting complex IV, potentiating reactive oxygen species (ROS) formation and decreasing ATP production. AD pathology can also be influenced by mutations in the mtDNA, since mtDNA from AD subjects have a higher rate of mutations. In Parkinson´s Disease (PD), complex I activity is impaired contributing to the formation of high levels of ROS. Many of the genes involved in PD are also associated with mitochondrial dysfunction. α-synuclein overexpression potentiates mitochondrial impairment and oxidative stress. Parkin associates with the outer mitochondrial membrane (OMM) protecting mitochondria against ROS release and caspase activation. DJ-1 is an integral protein that participates in the oxidative stress response and protects against the loss of dopaminergic neurons. In PD, the level of mtDNA mutations is also associated with respiratory chain deficiencies. In Huntington’s disease (HD), mutant huntingtin (htt) compromises complex II activity, ATP production and the calcium (Ca2+) buffering capacity. htt also affects mitochondrial function through its interaction with p53 in the nucleus leading to upregulation of BAX and PUMA, two pro-apoptotic proteins. In amyotrophic lateral sclerosis (ALS), mutant Cu/Zn superoxide dismutase (SOD1) that is localized in the outer mitochondrial membrane (OMM), intermembrane space (IMM) and mitochondrial matrix, impairs mitochondrial respiration and ATP synthesis as well as the mitochondrial Ca2+ loading capacity. Mutant SOD1 binds to Bcl-2 on the OMM blocking its anti-apoptotic activity.