Abstract
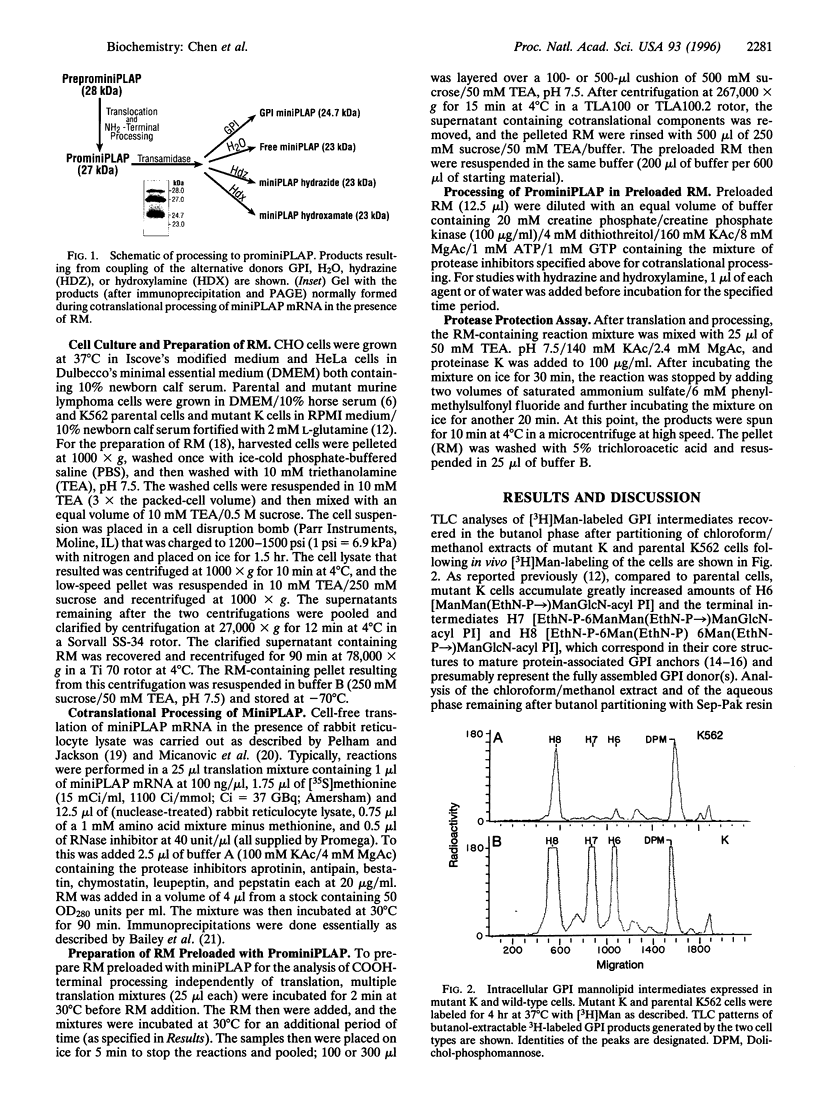
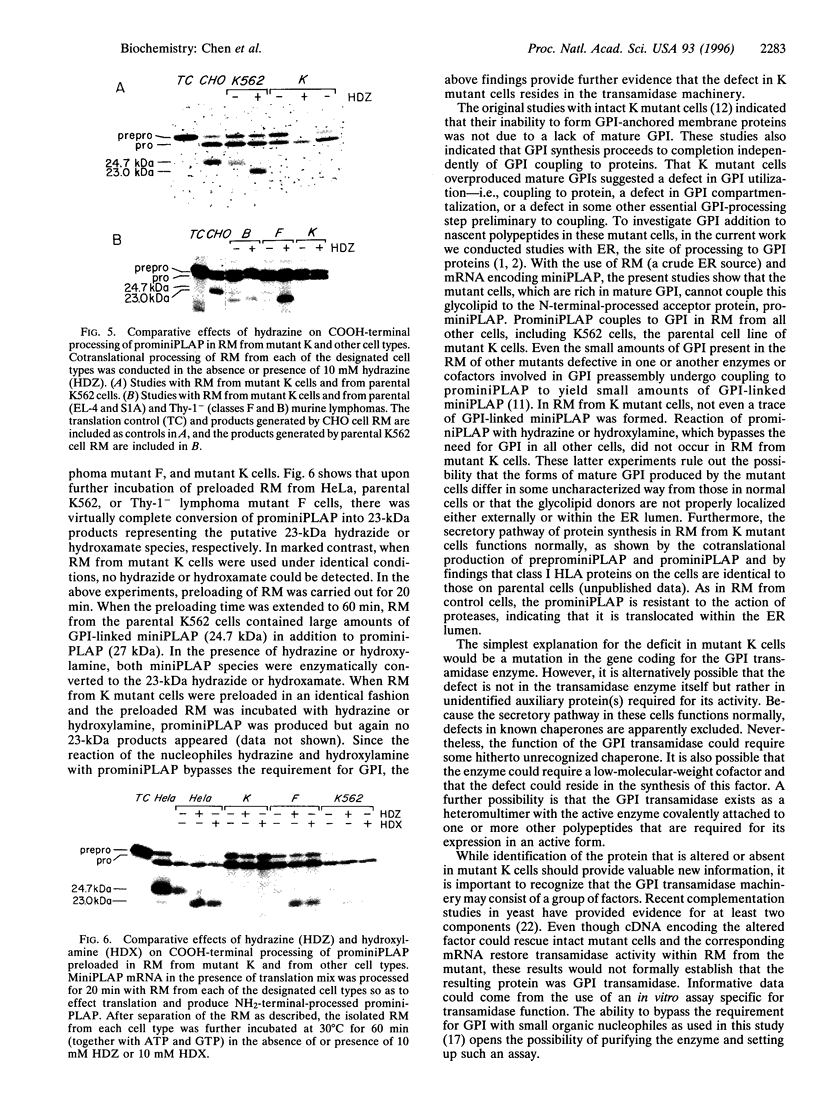
The final step in the pathway that provides for glycosylphosphatidylinositol (GPI) anchoring of cell-surface proteins occurs in the lumen of the endoplasmic reticulum and consists of a transamidation reaction in which fully assembled GPI anchor donors are substituted for specific COOH-terminal signal peptide sequences contained in nascent polypeptides. In previous studies we described a human K562 cell mutant line, designated class K, which assembles all the known intermediates of the GPI pathway but fails to display GPI-anchored proteins on its surface membrane. In the present study, we used mRNA encoding miniPLAP, a truncated form of placental alkaline phosphatase (PLAP), in in vitro assays with rough microsomal membranes (RM) of mutant K cells to further characterize the biosynthetic defect in this line. We found that RM from mutant K cells supported NH2-terminal processing of the nascent translational product, preprominiPLAP, but failed to show any detectable COOH-terminal processing of the resulting prominiPLAP to GPI-anchored miniPLAP. Proteinase K protection assays verified that NH2-terminal processed prominiPLAP was appropriately translocated into the endoplasmic reticulum lumen. The addition of hydrazine or hydroxylamine, which can substitute for GPI donors, to RM from wild-type or mutant cells defective in various intermediate biosynthetic steps in the GPI pathway produced large amounts of the hydrazide or hydroxamate of miniPLAP. In contrast, the addition of these nucleophiles to RM of class K cells yielded neither of these products. These data, taken together, lead us to conclude that mutant K cells are defective in part of the GPI transamidase machinery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. A., Gerber L., Howard A. D., Udenfriend S. Processing at the carboxyl terminus of nascent placental alkaline phosphatase in a cell-free system: evidence for specific cleavage of a signal peptide. Proc Natl Acad Sci U S A. 1989 Jan;86(1):22–26. doi: 10.1073/pnas.86.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghezal M., Lipke P. N., Conzelmann A. Identification of six complementation classes involved in the biosynthesis of glycosylphosphatidylinositol anchors in Saccharomyces cerevisiae. J Cell Biol. 1995 Sep;130(6):1333–1344. doi: 10.1083/jcb.130.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg M. A., Humphrey D. R., Yang S. H., Ferguson T. R., Reinhold V. N., Rosenberry T. L. Glycan components in the glycoinositol phospholipid anchor of human erythrocyte acetylcholinesterase. Novel fragments produced by trifluoroacetic acid. J Biol Chem. 1992 Sep 15;267(26):18573–18580. [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Hirose S., Prince G. M., Sevlever D., Ravi L., Rosenberry T. L., Ueda E., Medof M. E. Characterization of putative glycoinositol phospholipid anchor precursors in mammalian cells. Localization of phosphoethanolamine. J Biol Chem. 1992 Aug 25;267(24):16968–16974. [PubMed] [Google Scholar]
- Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W., Anand R., Williams A. F. Complete structure of the glycosyl phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein. Nature. 1988 May 19;333(6170):269–272. doi: 10.1038/333269a0. [DOI] [PubMed] [Google Scholar]
- Kamitani T., Menon A. K., Hallaq Y., Warren C. D., Yeh E. T. Complexity of ethanolamine phosphate addition in the biosynthesis of glycosylphosphatidylinositol anchors in mammalian cells. J Biol Chem. 1992 Dec 5;267(34):24611–24619. [PubMed] [Google Scholar]
- Kodukula K., Amthauer R., Cines D., Yeh E. T., Brink L., Thomas L. J., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: PI-G is an obligatory cosubstrate for COOH-terminal processing of nascent proteins. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4982–4985. doi: 10.1073/pnas.89.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Micanovic R., Gerber L., Tamburrini M., Brink L., Udenfriend S. Biosynthesis of phosphatidylinositol glycan-anchored membrane proteins. Design of a simple protein substrate to characterize the enzyme that cleaves the COOH-terminal signal peptide. J Biol Chem. 1991 Mar 5;266(7):4464–4470. [PubMed] [Google Scholar]
- Masterson W. J., Doering T. L., Hart G. W., Englund P. T. A novel pathway for glycan assembly: biosynthesis of the glycosyl-phosphatidylinositol anchor of the trypanosome variant surface glycoprotein. Cell. 1989 Mar 10;56(5):793–800. doi: 10.1016/0092-8674(89)90684-3. [DOI] [PubMed] [Google Scholar]
- Maxwell S. E., Ramalingam S., Gerber L. D., Udenfriend S. Cleavage without anchor addition accompanies the processing of a nascent protein to its glycosylphosphatidylinositol-anchored form. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1550–1554. doi: 10.1073/pnas.92.5.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Schwarz R. T., Mayor S., Cross G. A. Cell-free synthesis of glycosyl-phosphatidylinositol precursors for the glycolipid membrane anchor of Trypanosoma brucei variant surface glycoproteins. Structural characterization of putative biosynthetic intermediates. J Biol Chem. 1990 Jun 5;265(16):9033–9042. [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohney R. P., Knez J. J., Ravi L., Sevlever D., Rosenberry T. L., Hirose S., Medof M. E. Glycoinositol phospholipid anchor-defective K562 mutants with biochemical lesions distinct from those in Thy-1- murine lymphoma mutants. J Biol Chem. 1994 Mar 4;269(9):6536–6542. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Puoti A., Desponds C., Fankhauser C., Conzelmann A. Characterization of glycophospholipid intermediate in the biosynthesis of glycophosphatidylinositol anchors accumulating in the Thy-1-negative lymphoma line SIA-b. J Biol Chem. 1991 Nov 5;266(31):21051–21059. [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Stevens V. L., Raetz C. R. Defective glycosyl phosphatidylinositol biosynthesis in extracts of three Thy-1 negative lymphoma cell mutants. J Biol Chem. 1991 Jun 5;266(16):10039–10042. [PubMed] [Google Scholar]
- Sugiyama E., DeGasperi R., Urakaze M., Chang H. M., Thomas L. J., Hyman R., Warren C. D., Yeh E. T. Identification of defects in glycosylphosphatidylinositol anchor biosynthesis in the Thy-1 expression mutants. J Biol Chem. 1991 Jul 5;266(19):12119–12122. [PubMed] [Google Scholar]
- Ueda E., Sevlever D., Prince G. M., Rosenberry T. L., Hirose S., Medof M. E. A candidate mammalian glycoinositol phospholipid precursor containing three phosphoethanolamines. J Biol Chem. 1993 May 15;268(14):9998–10002. [PubMed] [Google Scholar]
- Urakaze M., Kamitani T., DeGasperi R., Sugiyama E., Chang H. M., Warren C. D., Yeh E. T. Identification of a missing link in glycosylphosphatidylinositol anchor biosynthesis in mammalian cells. J Biol Chem. 1992 Apr 5;267(10):6459–6462. [PubMed] [Google Scholar]