Abstract
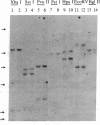
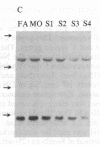
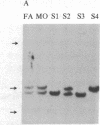
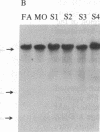
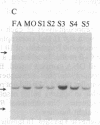
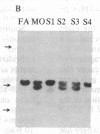
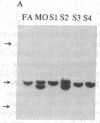
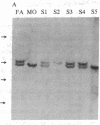
Polymorphism in the genes encoding the constant (C) region of the beta chain of the T-cell antigen receptor (CT beta, also called C beta) has been detected by molecular genotyping analyses. In initial screenings, a panel of restriction endonucleases was used to digest DNA samples from two individuals; the digested samples were subjected to Southern blot analyses using a CT beta probe. The enzyme Bgl II revealed restriction-fragment-length polymorphism in these samples and was subsequently used to test 59 individual members of eight different families. Polymorphic fragments detected in six of the families could be used to follow the segregation of T-cell receptor genes; in many cases maternal and/or paternal haplotypes could be assigned. All members of two additional families displayed a single CT beta hybridizing fragment. In one family the DNA sample from one of the children lacked an expected Bgl II restriction fragment. On the basis of analyses with other restriction enzymes, the most likely explanation is that the lymphoblastoid B-cell line used as a source of genomic DNA for this individual had rearranged or altered CT beta genes. Restriction-fragment-length polymorphisms used to discriminate CT beta haplotypes in families provide useful markers that will facilitate linkage studies and genetic analyses of T-cell function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Fabbi M., Smart J., Poole C. B., Protentis J., Royer H. D., Schlossman S. F., Reinherz E. L. Purification and NH2-terminal amino acid sequencing of the beta subunit of a human T-cell antigen receptor. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3851–3855. doi: 10.1073/pnas.81.12.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Ruddle F. H., Royer H. D., Acuto O., Reinherz E. L. Chromosomal location of human T-cell receptor gene Ti beta. Science. 1984 Oct 19;226(4672):348–349. doi: 10.1126/science.6435246. [DOI] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Goodfellow P. N., Dunne M. J., Spurr N. K., Solomon E., Owen M. J. A human T-cell antigen receptor beta chain gene maps to chromosome 7. EMBO J. 1984 Oct;3(10):2347–2349. doi: 10.1002/j.1460-2075.1984.tb02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A., Hobart M., Hengartner H., Rabbitts T. H. An immunoglobulin heavy-chain gene is altered in two T-cell clones. Nature. 1980 Aug 28;286(5776):897–899. doi: 10.1038/286897a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Hannum C. H., Kappler J. W., Trowbridge I. S., Marrack P., Freed J. H. Immunoglobulin-like nature of the alpha-chain of a human T-cell antigen/MHC receptor. Nature. 1984 Nov 1;312(5989):65–67. doi: 10.1038/312065a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Jones N., Leiden J., Dialynas D., Fraser J., Clabby M., Kishimoto T., Strominger J. L., Andrews D., Lane W., Woody J. Partial primary structure of the alpha and beta chains of human tumor T-cell receptors. Science. 1985 Jan 18;227(4684):311–314. doi: 10.1126/science.3871253. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Sherman D. H., Sitkovsky M. V., Pasternack M. S., Eisen H. N. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci U S A. 1984 Jan;81(2):573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. Biosynthesis and processing of murine T-cell antigen receptor. Cell. 1984 Oct;38(3):659–665. doi: 10.1016/0092-8674(84)90260-5. [DOI] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Robinson M. A., Long E. O., Johnson A. H., Hartzman R. J., Mach B., Kindt T. J. Recombination within the HLA-D region. Correlation of molecular genotyping with functional data. J Exp Med. 1984 Jul 1;160(1):222–238. doi: 10.1084/jem.160.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yanagi Y., Suciu-Foca N., Minden M., Mak T. W. Rearrangements of T-cell receptor gene YT35 in human DNA from thymic leukaemia T-cell lines and functional T-cell clones. 1984 Sep 27-Oct 3Nature. 311(5984):385–387. doi: 10.1038/311385a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]