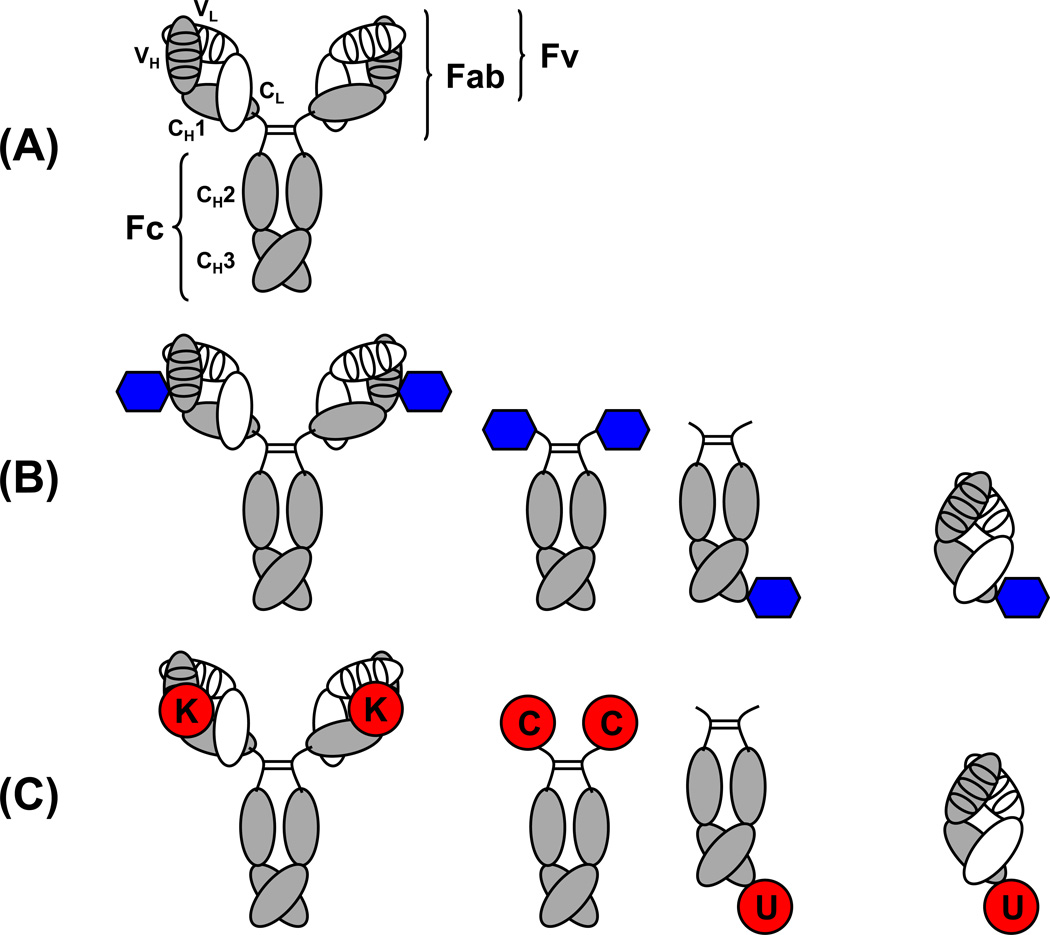
Figure 1. Comparison of the architecture of conventional mAbs and cpAbs.
(A) The 150-kDa IgG1 molecule is the dominantly used pharmaceutical format of mAbs. It contains two identical light chains (white) and two identical heavy chains (gray). The light chains consist of an N-terminal variable domain (VL) followed by one constant domain (CL). The heavy chains consist of an N-terminal variable domain (VH) followed by three constant domains (CH1, CH2, and CH3). CH1 and CH2 are linked through a flexible hinge region (bent lines) that anchors four interchain disulfide bridges of the IgG1 molecule, one for each of the two light and heavy chain pairs (not shown) and two for the heavy chain pair (straight lines). The antigen binding site, or paratope, is formed by six CDRs (ovals), three provided by each variable domain. Fv, Fab, and Fc fragments of the IgG1 molecule are indicated. (B) Antigen binding in cpAbs is mediated by a synthetic component (blue hexagon) that is site-specifically and covalently attached to an antibody component. The antibody component of cpAbs typically is a whole IgG1 molecule, which displays two synthetic components (left), or a symmetric or asymmetric Fc fragment, which displays two or one, respectively, synthetic components on either its N- or C-terminus (center). In contrast to these IgG-based and Fc-based cpAbs, Fab-based cpAbs (right) bind one antigen with their synthetic component and another antigen with their antibody component. (C) Unique reactivity centers in the antibody component of cpAbs include a reactive lysine (K) residue in the paratopes of an IgG (left), engineered N-terminal cysteine (C) or engineered C-terminal selenocysteine (U) residues of a Fc fragment (center), and an engineered C-terminal selenocysteine (U) of a Fab fragment (right).