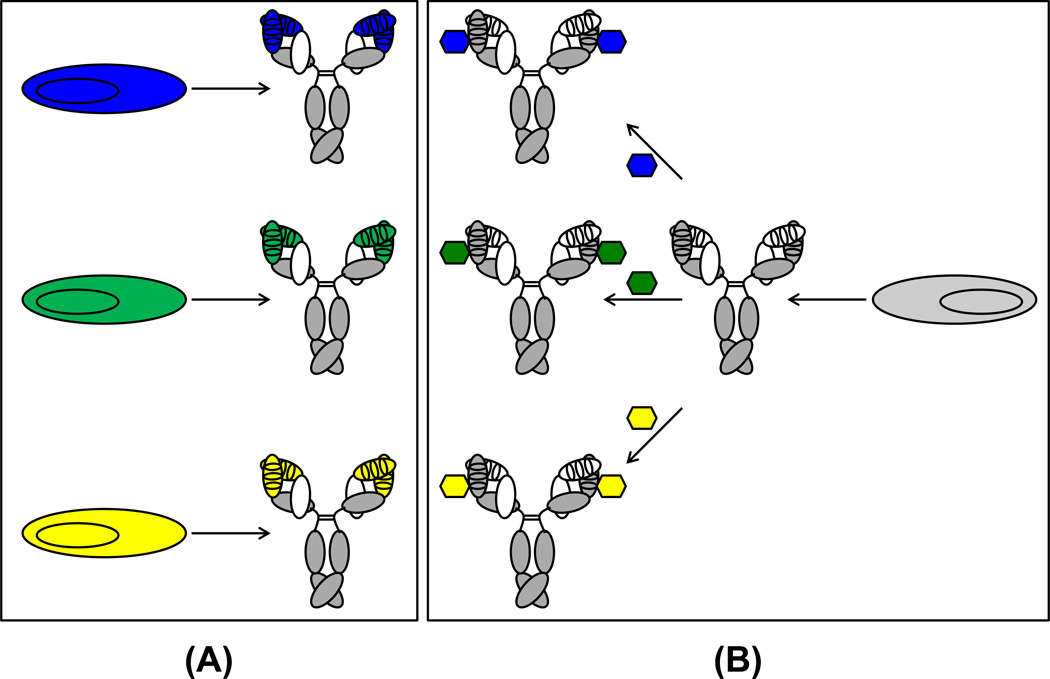
Figure 2. Comparison of the production of conventional mAbs and cpAbs.
(A) In conventional mAbs, antigen binding is mediated by the paratope, requiring the cloning, expression, and purification of different proteins for different specificities (blue, green, and yellow). (B) By contrast, cpAbs only require the manufacturing of a single protein (i.e., the antibody component), which is then chemically programmed with different small molecules (i.e., the synthetic components) for targeting different specificities. Since GMP manufacturing costs are substantially lower for small molecules compared to proteins, cpAbs are economically more attractive than conventional mAbs. Using the same protein for a range of specificities and indications also shortens drug discovery and development time.