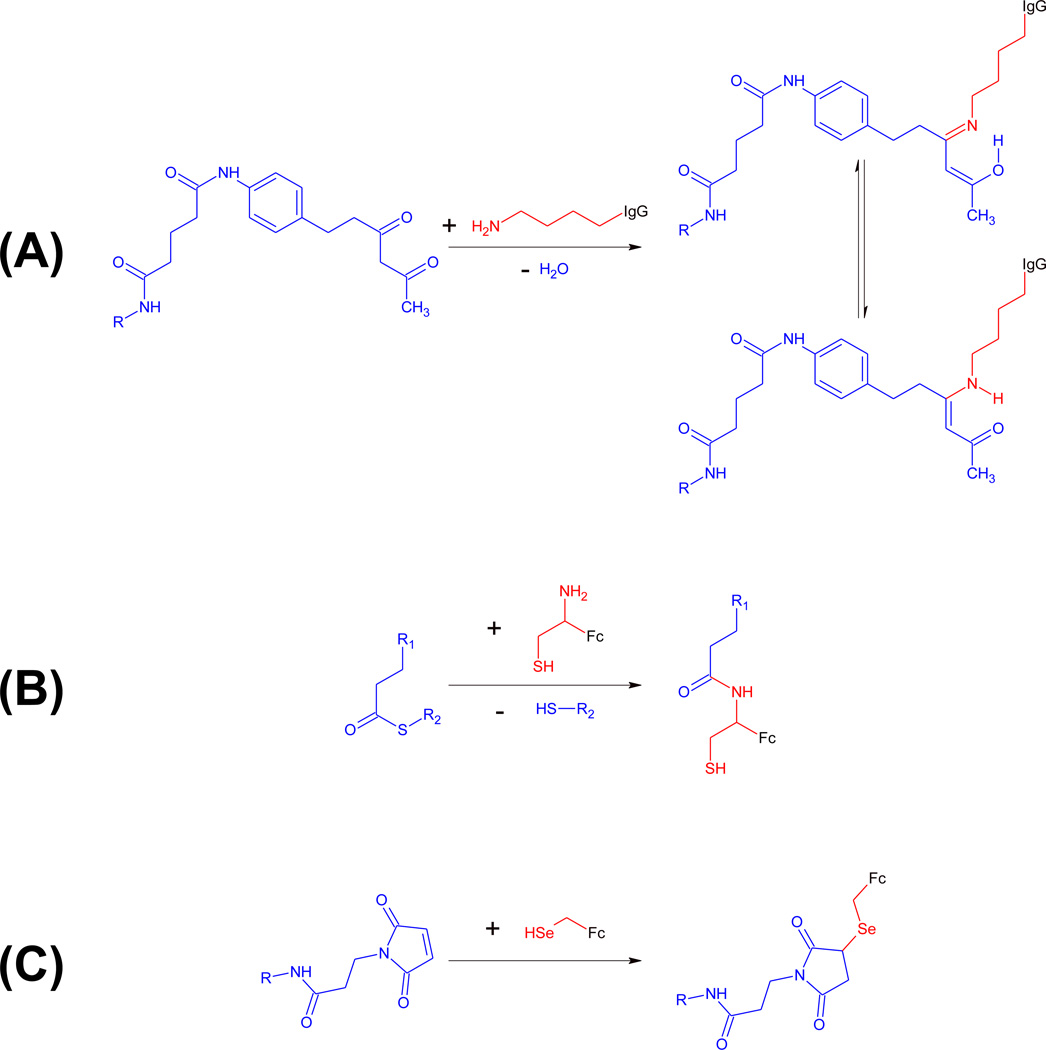
Figure 3. Covalent conjugation in cpAbs.
Unique reactivity centers of antibody components are shown in red, synthetic components are shown in blue. (A) mAbs 38C2 and h38C2 harbor a reactive lysine residue (red) at the base of a deep hydrophobic cleft. The nucleophilic ε-amino group of this reactive lysine residue can be covalently conjugated to small molecules derivatized with an electrophilic 1,3-diketone group (blue). The covalent adduct is stabilized by imine-enamine tautomerism. (B) Fc fragments with an engineered N-terminal cysteine display a naturally occuring 1,2-aminothiol group (red), which reacts with small molecules derivatized with a thioester group (blue) in the presence of sodium 2-mercaptoethanesulfonate (MESNA) by first undergoing transthioesterification and then S-to-N acyl shift rearrangement under formation of an amide bond. (C) Fc fragments with an engineered C-terminal selenocysteine display a selenol group (red), which reacts with maleimide (blue) or iodoacetamide derivatives of small molecules.