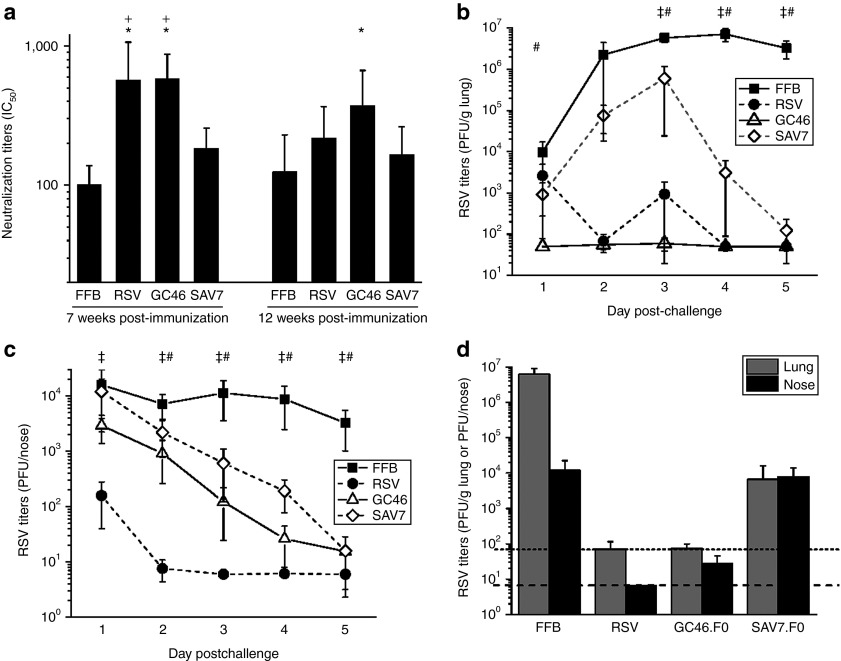
Figure 7.
GC46.F0 induces protective immunity rapidly, clears RSV infection, and is durable. Mice were immunized intramuscularly with 106 PFU RSV A2, 108 particle units (PU) GC46.F0, or 108 PU SAV7.F0. (a) Serum was obtained at 7 and 12 weeks postimmunization, and neutralizing IC50 titers determined by plaque reduction neutralization test assay. N = 10 mice per group at each time point. *Statistically different from FFB-immunized mice; +Statistically different from SAV7.F0-immunized mice by one-way analysis of variance (ANOVA). (b,c) A subset of mice was challenged with 5 × 106 PFU RSV A2 at 7 weeks postimmunization. Mice were euthanized at days 1, 2, 3, 4, and 5 postchallenge, and RSV titers in the (b) lung and (c) nose were measured by plaque assay. N = 5 mice per group per time point, and limits of detection are 70 PFU/g lung and 6.7 PFU/nose. Statistical significance was determined by one-way ANOVA, where P < 0.05. ‡Statistically difference between FFB- and RSV-immunized mice; #Statistically difference between FFB-immunized mice and GC46.F0- or SAV7.F0-immunized mice. (d) A subset of mice was challenged with 5 × 106 PFU RSV A2 at 12 weeks postimmunization, then euthanized at day 5 postchallenge. RSV titers in the lung and nose were determined by plaque assay. Limits of detection are 70 PFU/g lung and 6.7 PFU/nose as denoted by dotted and dashed lines, respectively. N = 5 mice per group. FFB, final formulation buffer; PFU, plaque-forming units; RSV, respiratory syncytial virus.