Figure 2.
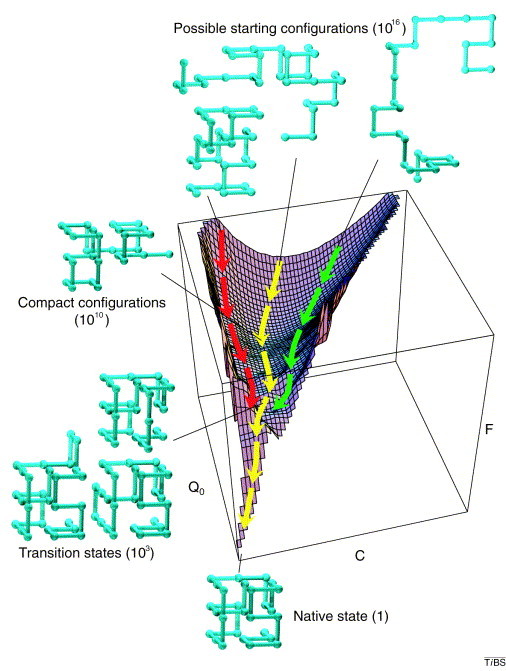
Simple folding surface. Free-energy (F) surface of a 27-residue model protein. The axes are the number of native contacts (Q0) and the total number of (native and non-native) contacts (C). The yellow trajectory shows the average path traced by the unfolded protein. The green and red trajectories lie two standard deviations from the average; thus 95% of all trajectories would be expected to exist within this range. The green structures outside the axes illustrate the various stages of the reaction. A folding peptide collapses rapidly from one of its 1,016 possible random starting conformations to a disordered globule. It then makes a slow, non-directed search among the 1,010 semi-compact conformations for one of the approximately 103 transition states that lead rapidly to the unique native state. Adapted from [51].
