Figure 5.
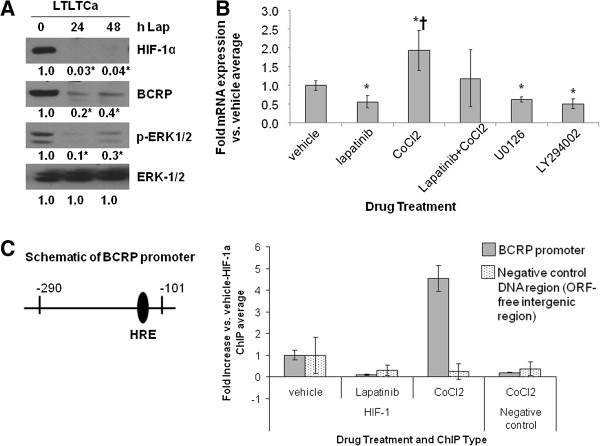
Effect of lapatinib and CoCl2 on BCRP protein and mRNA expression and on HIF-1α binding to the BCRP promoter. A-B), A) LTLTCa cells were treated with 1 μM lapatinib for 0 to 48 hours. Total protein was extracted and underwent Western blot for HIF-1α, BCRP, p-ERK1/2, and ERK. Shown are representative blots and overall densitometry results of n = 6 independent cell samples/group. Densitometry results are expressed as mean fold-change compared to 0 hours after normalization to ERK (mean ± SD of n = 6 independent cell samples/group; *versus 0 hours lapatinib; P = 0.0004 for ERK for HIF-1α, P = 0.0017 for BCRP, P = 0.0009 for phospho-ERK1/2, P = 1 for ERK-1/2; one-way ANOVA). B) Total RNA was extracted and underwent real-time RT-PCR for BCRP mRNA and 18S rRNA. Real-time results are the mean fold-change in mRNA levels compared with vehicle after normalization to 18S rRNA (mean ± SD of n = 6 independent cell samples/group; *versus vehicle, P <0.05; † versus lapatinib, U0126 or LY294002, P <0.01; overall P <0.0001, one-way ANOVA). C) LTLTCa were treated with vehicle, 1 μM lapatinib, and/or 100 μM CoCl2 for 24 hours. Protein-DNA complexes from LTLTCa cells were analyzed by ChIP analysis. Immunoprecipitation was done either with HIF-1α antibody or an equivalent volume of normal mouse serum (negative control). Primers for either the -290 to -101 region of the human BCRP promoter, which contains the HRE to which HIF-1 binds, or ORF-free intergenic region (negative control DNA region) were used for real-time PCR. Results are the mean fold increase compared with vehicle-treated cells after normalization to input samples of each (means ± SD, n = 6 independent cells sample/group; *versus vehicle-HIF-1 IP; P = 0.004 for BCRP promoter, P = .2972 for negative control ORF-free intergenic region; one-way ANOVA). ANOVA, analysis of variance; BCRP, breast cancer resistant protein; ChIP, chromatin immunoprecipitation; HIF-1α, hypoxia inducible factor 1 α subunit; n, number; SD, standard deviation.
