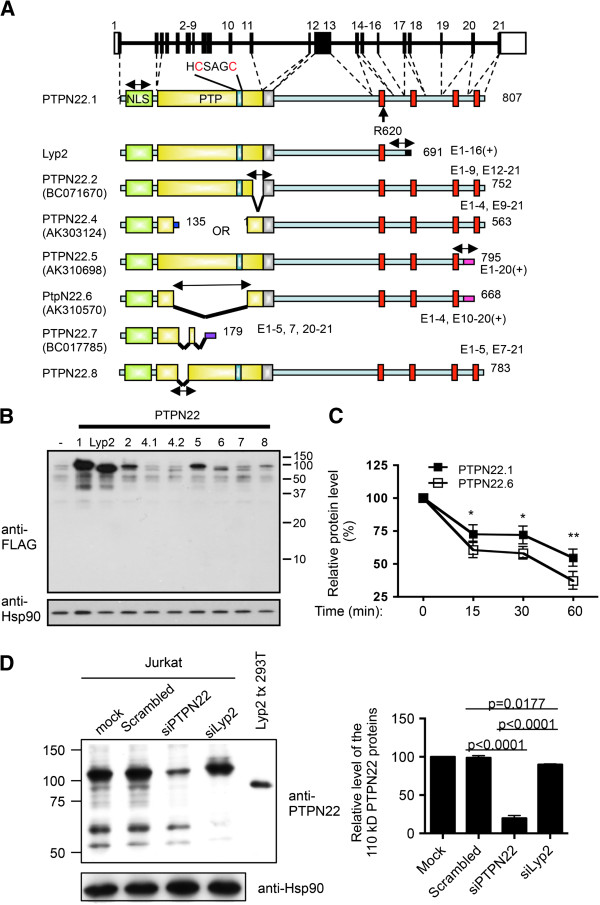
Figure 1.
Alternatively spliced forms of human protein tyrosine phosphatase, non-receptor type 22 (PTPN22). (A) Schematic diagrams of the human ptptn22 gene and putative protein products of each isoform. Exons are numbered, and white boxes represent untranslated exons. Green boxes represent nuclear localization signal (NLS). Yellow boxes represent the protein tyrosine phosphatase (PTP) domains. The key catalytic region (HCSAGC) and R620 are marked. Silver boxes are inhibitory domains. Red boxes represent proline-rich regions. Two-headed arrows indicate the real-time PCR products of the isoforms. The exons constituting each isoform are indicated. The GenBank accession numbers, if available, are listed in parentheses. (B) 293 T cells were transfected with 1 μg of an expression vector expressing indicated FLAG-PTPN22 isoforms. The protein levels of FLAG-PTPN22 isoforms and Hsp90 in the transfected cells were determined with Western blotting. (C). 293 T cells were transfected with a plasmid vector expressing FALG-PTPN22.1 and FLAG-PTPN22.6. The transfected cells were treated with 20 ug/ml of cycloheximide. The levels of FLAG-PTPN22.1 and FLAG-PTPN22.6 were then determined with Western blotting and densitometry at indicated time points after the addition of cycloheximide. The level at time 0 for each protein was arbitrarily set as 100%. Statistical significance was determined with paired Student’s t test. *P <0.05; **P <0.005. (D) Jurkat cells were transfected with indicated siRNA. Cell extract of the transfect cells was then analyzed on Western blotting using anti-PTPN22 and anti-Hsp90 (the left panel). Extract from 293 T cells transfected with an expression vector of Lyp2 was included in the Western blotting (Lyp2 tx 293 T). The levels of the dominant 110 kD PTPN22 protein bands were quantified with a densitometer and normalized against the level of Hsp90 from the corresponding samples, and are shown in the right panel. The normalized level of the mock-transfected cells was arbitrarily set as 100%. Statistical analysis of three independent experiments was performed with one-way ANOVA followed by Tukey’s test.