Figure 3.
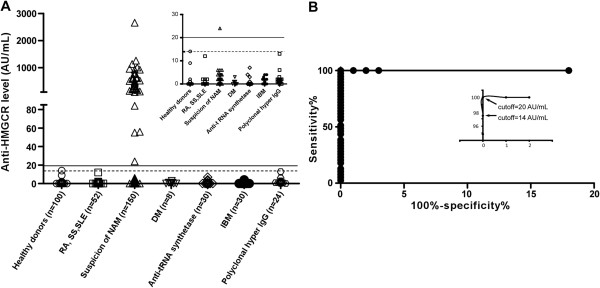
Validation of ALBIA-HMGCR and determination of anti-HMGCR levels in patients with suspicion of NAM. (A) A positivity cutoff of the assay was first determined as the 99th percentile (14 arbitrary units (AU)/mL) of the healthy donors’ distribution (open circles) and depicted by a dotted line. Sera from patients with different inflammatory/autoimmune conditions including rheumatoid arthritis (RA), systemic sclerosis (SS), systemic lupus erythematosus (SLE), dermatomyositis (DM), anti-tRNA synthetase-positive myositis or inclusion body myositis (IBM), as well as patients with polyclonal hypergammaglobulinemia were assayed and revealed all negative. Insert is a magnification for low values. Thirty-seven out of 150 (24%) of serum samples from patients with suspicion of NAM scored positive using ALBIA-HMGCR. (B) Receiver operating characteristic (ROC) analysis was performed by comparing the 37 anti-HMGCR positive serum samples to those from healthy donors. Optimal results were obtained with a cutoff of 20 AU/mL, which was indicated as a plain line. ALBIA, addressable laser bead immunoassay; HMGCR, 3-hydroxy-3-methylglutaryl coenzyme A reductase; NAM, necrotizing autoimmune myopathies.
