Figure 5.
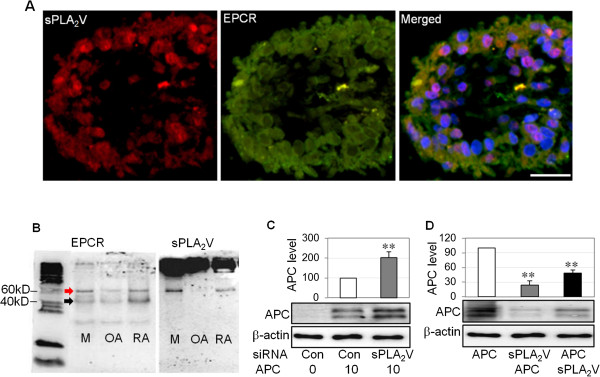
Group V secretory phospholipase A2 (sPLA2V) is co-localized with endothelial protein C receptor (EPCR) and blocks activated protein C (APC) binding to rheumatoid synovial fibroblasts (RASFs). (A) sPLA2V was co-localized with EPCR in rheumatoid arthritis (RA) synovial tissue, detected by immunofluorescent staining. Nuclei were counterstained by 4′-6-diamidino-2-phenylindole (DAPI) (blue). Scale bar: 50 μm. (B) sPLA2V and EPCR expression by MCF-7 cells (M, used as a positive control), RASFs (RA), or OASFs (OA), detected by immunoprecipitation using anti-EPCR antibody and followed by Western blot using anti-EPCR or sPLA2V antibody under non-reducing conditions. Red arrow indicates EPCR and sPLA2V complex. Black arrow indicates EPCR. (C) APC in whole cell lysates of RASFs transfected with small interfering RNA (siRNA) for control (Con) or sPLA2V siRNA for 48 hours and treated with APC (10 μg/mL) for 24 hours and detected by Western blot. (D) APC in the whole cell lysates of RASFs treated with recombinant APC (2 μg/mL), recombinant sPLA2V (2 μg/mL) for first 30 minutes then APC (sPLA2V + APC) or APC for first 30 minutes then sPLA2V (APC + sPLA2V) for 4 hours, detected by Western blot. The images represent one of three different experiments using three different RASF cell lines. Data on (C) and (D) were semi-quantified by image analysis software in comparison with β-actin, expressed as a percentage of control and shown as mean ± standard deviation (SD) (n = 4) and analyzed by one-way analysis of variance followed by Tukey’s honestly significant difference (HSD) post hoc test. **P <0.01.
