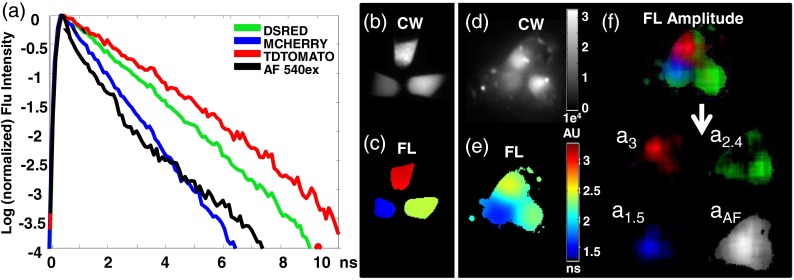
Fig. 4.
TD FL multiplexing enables the separation of three red shifted fluorescent proteins (RFPs) from mouse skin autofluorescence. Measurements of pelleted RFPs were made in vitro before and after placement under the skin of a sacrificed nude mouse. (a) tdTomato, dsRed, and mCherry have distinct, single exponential fluorescence decays with corresponding lifetimes of 3, 2.4, and 1.5 ns, respectively, while mouse skin AF (543-nm excitation) is best fit by a biexponential decay (lifetimes 0.27 and 2.1 ns). (b) CW intensity provides no identifying contrast between the tubes containing the three RFPs. (c) A single exponential fit of the fluorescence TPSF correctly reveals the lifetime of the FP expressed in each tube. (d) CW image cannot distinguish tubes containing the three RFPs placed under the skin of a nude mouse. (e) Lifetime map of the tubes under the mouse skin from a single exponential fit to each pixel. The effect of tissue AF leads to incorrect lifetimes for the tubes. (f) The contribution of each source of fluorescence can be determined by performing a multiexponential fit of the total fluorescence TPSF with four basis functions: one corresponding to the biexponential decay of the skin AF, and three single exponential decays corresponding the known FL of each RFP. For visualization, the resulting FL amplitudes: , , and are shown in red, green, and blue, respectively, of a single RGB image. Here, the FL amplitude corresponding to each FP correctly highlights and identifies the contents of each tube. The tissue AF amplitude distribution is shown in grayscale.