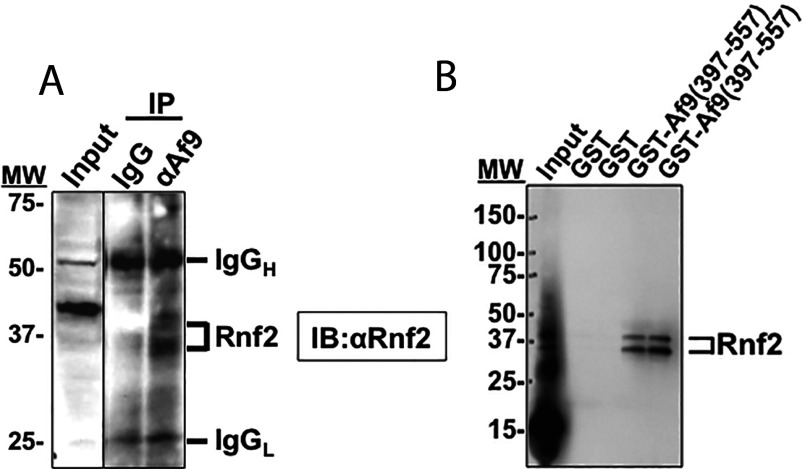
Figure 1. Rnf2 and Af9 interact in vitro and in vivo.
(A) Co-immunoprecipitation assay demonstrating that endogenous Rnf2 and Af9 proteins in mIMCD3 cells are present in the same protein complex. Whole cell lysates of mIMCD3 cells were IP (immunoprecipitated) with anti-Af9 antibody or IgG (as a negative control) as detailed in ‘Materials and Methods.’ IP proteins were further resolved on IBs (immunoblots) probed with anti-Rnf2 antibody. The IB is representative of three independent experiments. Positions of MW (molecular weight) markers are indicated. The Rnf2 doublet likely reflects unmodified and autoubiquitinated forms of endogenous Rnf2 [23]. (B) GST pull-down assay showing interaction of in vitro translated Rnf2 with amino acids 397–557 of Af9. GST and GST–Af9-(397–577) fusion protein were purified from E. coli and incubated with in vitro translated Rnf2. Proteins bound to glutathione-Sepharose 4B beads were examined by IB analysis with anti-Rnf2 antibody (n=3). Positions of MW markers are indicated. The Rnf2 doublet likely reflects unmodified and autoubiquitinated forms of Rnf2 [23] generated during in vitro translation with the reticulocyte lysate system [24].