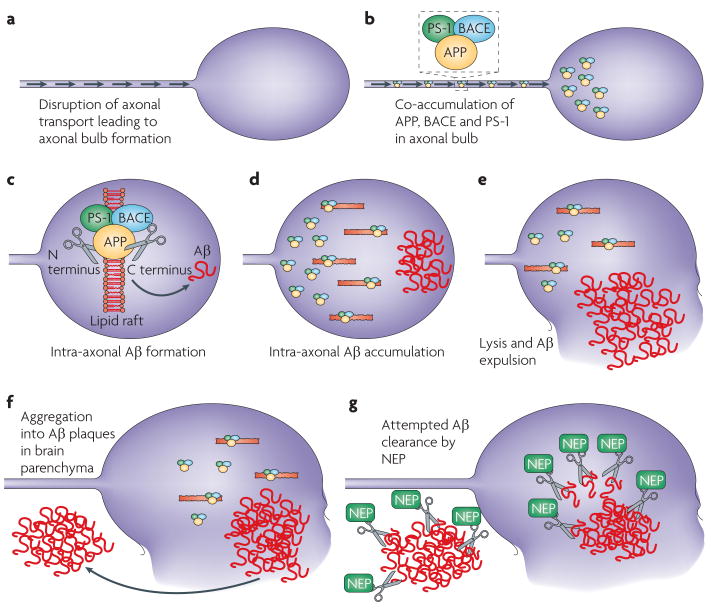
Figure 2. Potential mechanisms of post-traumatic amyloid-β formation and clearance.
a | The mechanical forces that axons are subjected to during a traumatic event can damage axons by directly altering their structure or by initiating detrimental secondary cascades. Failure of axonal transport in these injured axons results in accumulation of multiple proteins that form swellings at their disconnected terminals known as axon bulbs. b | such protein accumulation has been demonstrated to include the enzymes necessary for the cleavage of amyloid precursor protein (APP) to amyloid-β (Aβ), including presenilin-1 (PS-1) and β-site APP-cleaving enzyme (BACE). c–d | Although the precise intracellular mechanism of Aβ genesis remains unclear, lipid rafts have been suggested to be important in allowing APP processing and thus Aβ accumulation within the axonal compartment. e–f | Injured axons that go on to degenerate and lyse will expel the accumulated Aβ into the brain parenchyma where it is at risk of aggregating into plaques. g | The enzyme that clears Aβ, neprilsyin (NeP), also accumulates in damaged axons and probably mitigates the effects of enhanced Aβ production. The balance of genesis versus catabolism will ultimately determine Aβ build-up. NeP may potentially act to clear Aβ within the axonal compartment or in the extracellular space.