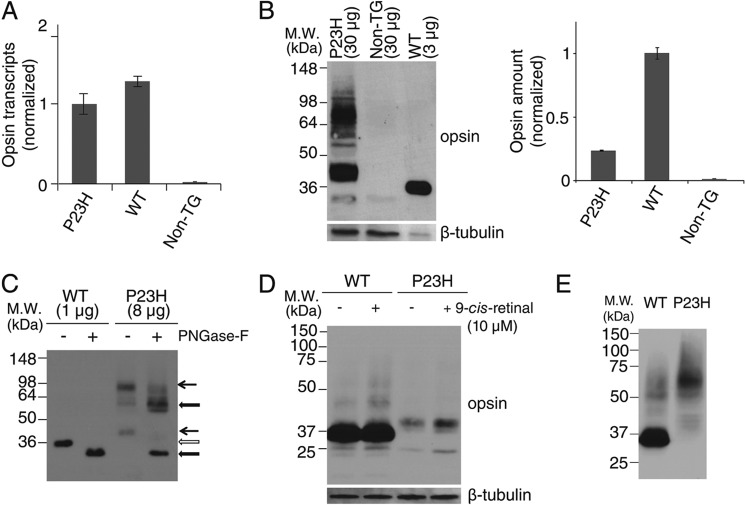
FIGURE 2.
Characterization of P23H opsin expression in C. elegans. A, comparison of opsin transcripts expressed in WT and P23H opsin worms normalized with β-tubulin. B, immunoblots of bovine opsin in stable C. elegans expressing WT opsin or P23H opsin with non-TG worms used as a control. β-Tubulin was the loading control. Because of the low abundance of P23H opsin in each worm, 30 μg of worm lysate protein were loaded for P23H TG worm lines, but only 3 μg of lysate were used to generate a comparable WT band. Quantified band intensities normalized by β-tubulin are shown in the right panel. C, immunoblots of WT opsin and P23H opsin from nematode lysates with or without peptide:N-glycosidase F (PNGase-F) treatment for deglycosylation. Positions of the bands for untreated WT (open arrow) and P23H (black arrow) opsin are indicated. The difference in band positions between WT opsin and P23H opsin was due to altered glycosylation because the bands of deglycosylated WT opsin and P23H opsin occupied similar positions after treatment with peptide:N-glycosidase F (thick black arrow). D, effect of 9-cis-retinal pretreatment on WT opsin and P23H nematode opsin. Freshly prepared samples were immediately loaded on SDS-PAGE. Both WT (∼35 kDa) and P23H opsin (∼40 kDa) were observed as monomers. β-Tubulin was used as a loading control. E, immunoblots of WT and P23H opsin from worm lysates frozen at −80 °C for 1 day. Worms were homogenized and frozen immediately for SDS-PAGE on the next day. P23H opsin was present primarily as a dimer at ∼70 kDa, whereas WT opsin existed primarily as a monomer at ∼35 kDa.