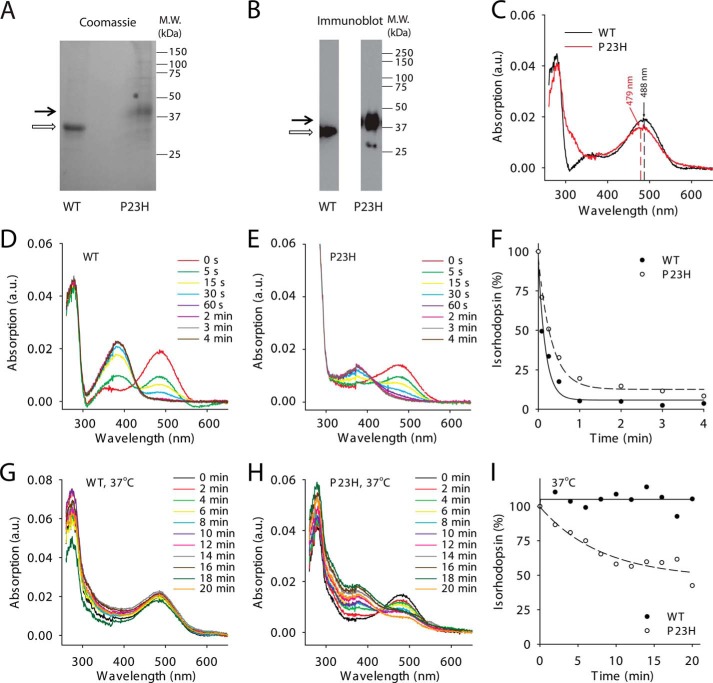
FIGURE 4.
Biochemical characterization of WT and P23H isorhodopsins purified from C. elegans. Purified WT isorhodopsin and P23H isorhodopsin in Coomassie Blue-stained SDS-polyacrylamide gels (A) and immunoblots (B) are indicated by open and black arrows, respectively. C, absorption spectra of WT (black line) and P23H (red line) isorhodopsin. The maximum absorptions of WT isorhodopsin and P23H isorhodopsin were at 487.5 and 478.8 nm, respectively. D and E show photosensitivity assay results for WT isorhodopsin and P23H isorhodopsin, respectively. Absorption spectra were taken from isorhodopsin illuminated by a fiber light through a bandpass filter (480–520 nm) for different periods of time. F, comparison of WT (solid circles) and P23H (open circles) isorhodopsin photosensitivities plotted as a function of illumination time in minutes. Isorhodopsin concentrations were measured by absorption at 488 nm for WT and at 479 nm for P23H isorhodopsin and normalized to the isorhodopsin concentration before illumination. G and H, absorption spectra of WT isorhodopsin (G) and P23H isorhodopsin (H) after incubation at 37 °C for specified amounts of time reflect their thermal stability. I, thermal stability of WT isorhodopsin (solid circles) and P23H isorhodopsin (open circles) was compared by plotting the percentage of isorhodopsin remaining as a function of time at 37 °C, normalized by their initial concentrations.