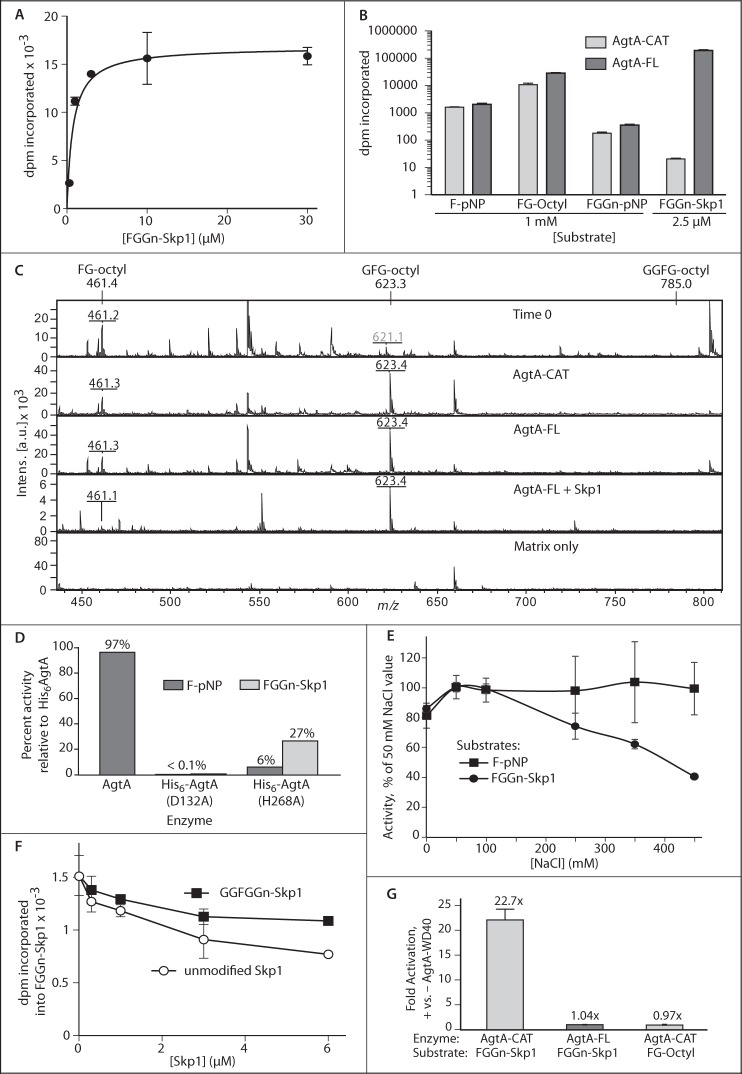
FIGURE 2.
Domain dependence of AgtA α-GalT activity. A, His6AgtA-FL was incubated with a series of FGGn-Skp1 concentrations in the presence of 40 μm UDP-[3H]Gal and 50 mm NaCl for 30 min. Incorporation of [3H]Gal was quantified by TCA precipitation. The curve was derived by non-linear regression according to the Michaelis-Menten model. B, His6AgtA-FL or His6AgtA-CAT was incubated in the presence of 10 μm UDP-[3H]Gal, 50 mm NaCl, and the indicated acceptor substrate for 30 min. Incorporation was quantitated by Sep-Pak C18 capture (pNP and octyl aglycons) or TCA precipitation (Skp1). His6AgtA-CAT protein concentration was 10-fold higher than that of His6AgtA-FL to yield similar activity toward F-pNP. Results are reported as the mean of triplicate reactions ± S.D. C, MALDI-TOF-MS of the reaction of His6AgtA-FL or His6AgtA-CAT with FG-octyl. Expected and experimental m/z values ((M + Na+)+) for FG-octyl and the potential products GFG-octyl and GGFG-octyl are indicated. Other ions represent contaminants, which are common in the low mass range for MALDI-TOF-MS experiments. D, activities of AgtA (tag removed), His6AgtA(D132A), and His6AgtA(H268A), relative to full-length His6AgtA, toward substrates F-pNP and FGGn-Skp1 were determined as in panel B. E, salt dependence of α-GalT activity of AgtA-FL toward F-pNP or FGGn-Skp1 was assayed as in B. Average values ± S.E. of triplicate reactions are reported. F, effect of non-substrate isoforms of Skp1 on AgtA activity, measured as transfer of [3H] from UDP-[3H]Gal to 0.25 μm FGGn-Skp1. Error bars represent ± S.D. for assays conducted in triplicate, which are representative of 3 independent trials. G, α-GalT reactions with 0.15 μm FGGn-Skp1 or 840 μm FG-octyl were supplemented with 0.31 μm His6AgtA-WD40, and the results are reported as fold-activation relative to activity in its absence. Data are reported as representative mean ± S.D.