FIGURE 7.
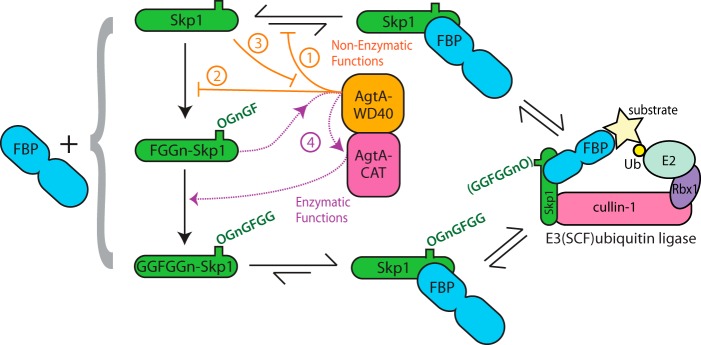
Proposed impact of AgtA on Skp1 interactions. Progressive Skp1 glycosylation is shown at the left from top to bottom. Each Skp1 isoform can bind to an F-box protein (FBP, depicted at left), but evidence (25) indicates that FBPs prefer glycosylated Skp1s, which are reflected by the equilibria shown at the top and bottom. The Skp1·FBP complex ultimately enters the E3SCF·ubiquitin ligase complex shown at the right. AgtA, in the center, is both a Skp1-binding protein and the enzyme that α-galactosylates FGGn-Skp1 to yield GGFGGn-Skp1. We propose that AgtA, via its WD40 repeat domain (in orange), competes with FBP binding to Skp1 (step 1), and competes with recognition of Skp1 by PhyA and Gnt1 (step 2), thereby inhibiting Skp1 function and processing in cells. This interaction in turn titrates AgtA from its later catalytic role (step 3). FBP binding also interferes with Skp1 hydroxylation by PhyA (7). Once Skp1 is modified to the trisaccharide state as FGGn-Skp1, its second-site, salt-sensitive recognition by the WD40 repeat domain of AgtA activates the catalytic activity of AgtA (purple) resulting in switch-like α-galactosylation of Skp1 (step 4).
