FIGURE 1.
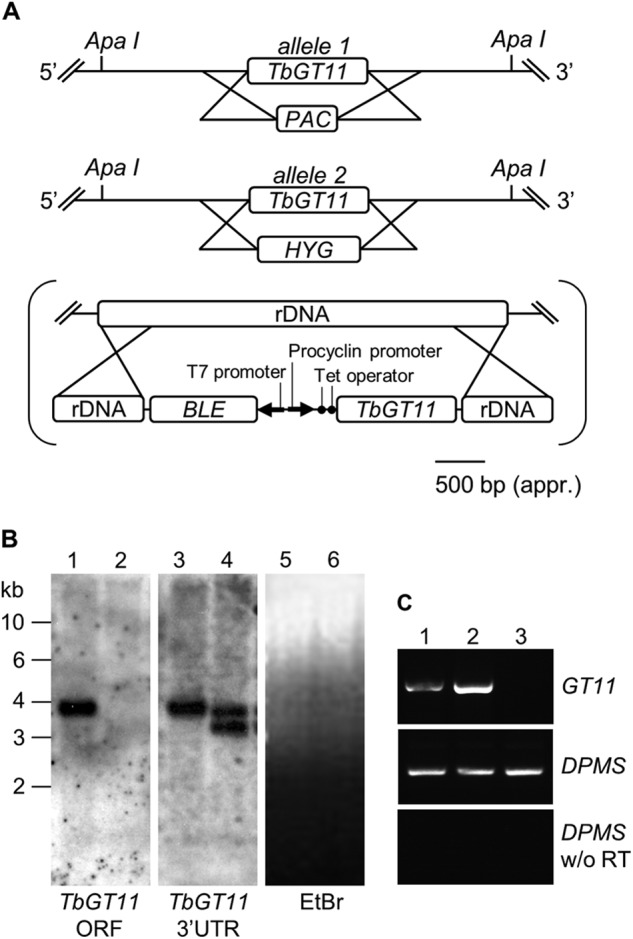
Generation of a bloodstream-form TbGT11 null and conditional mutant. A, gene replacement strategy to create TbGT11 null mutant cells and subsequent insertion of tetracycline-inducible ectopic copy, in brackets, to create a conditional null mutant. B, Southern blot of genomic DNA digested with ApaI from WT (lanes 1, 3, and 5) and TbGT11 null mutant cells (lanes 2, 4, and 6). The blot was probed with a TbGT11 ORF probe (left hand panel) and a TbGT11 3′ UTR probe (middle panel) and indicates the replacement of both alleles with drug resistance cassettes. Equal loading was verified by ethidium bromide staining (right hand panel). C, ethidium bromide-stained agarose gel of reverse transcription-PCR products from RNA extracted from WT cells (lane 1) and TbGT11 conditional null mutants grown under permissive (lane 2) or non-permissive conditions (lane 3). The upper panel shows RT-PCR products using primers for TbGT11, the middle panel is a control using dolicholphosphate mannose synthase (DPMS) primers to show equal RNA input, and the lower panel is a control without reverse transcriptase.
