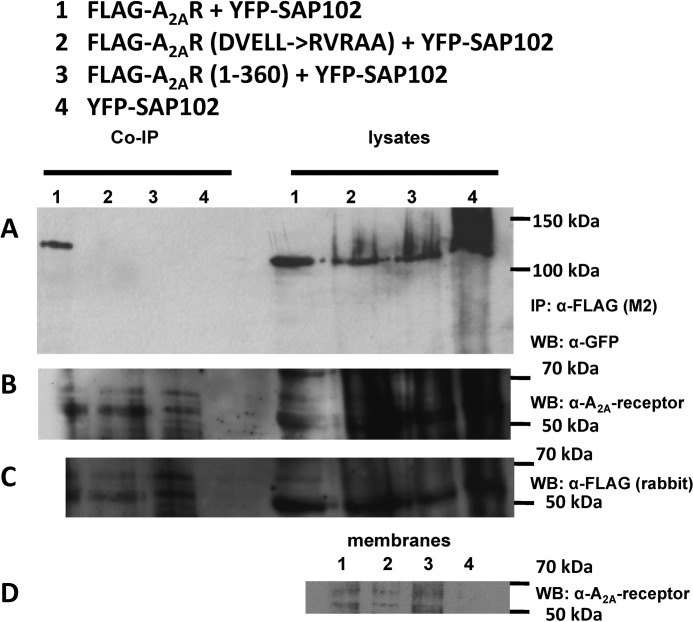
FIGURE 4.
Coimmunoprecipitation of SAP102 with wild-type and mutated versions of the A2A receptor. A, detergent lysates were prepared from HEK293 cells (1 × 106 cells containing 0.53 ± 0.08, 0.45 ± 0.09, and 0.50 ± 0.10. pmol receptors for wild-type A2A receptor, A2A receptor-RVRAA, and A2A receptor(1–360), respectively, as assessed by binding of [3H]ZM241385) coexpressing YFP-tagged SAP102 with FLAG-tagged versions of the wild-type A2A receptor (lane 1), the A2A receptor-383RVRAA387, a version with a mutated DVELL motif (lane 2), and the truncated A2A receptor(1–360) (lane 3) or cotransfected with an empty plasmid (lane 4) and incubated with anti-FLAG M2 affinity matrix. Bound proteins were released by heat denaturation in SDS-containing reducing sample buffer. The immunoprecipitated material was electrophoretically resolved together with an aliquot (20 μg) of the corresponding lysate, transferred to nitrocellulose, and probed with an antiserum directed against GFP (A). IP, immunoprecipitation; WB, Western blot. The nitrocellulose blots were also probed with a monoclonal antibody directed against the A2A receptor (epitope in the third intracellular loop, B) or a rabbit polyclonal antiserum raised against the FLAG epitope (C). Note that the immunoreactive bands corresponding to the various glycosylated forms of the A2A receptor can only be detected in the immunoprecipitate but not in the lysates. Membranes (20 μg) prepared from HEK293 cells coexpressing YFP-tagged SAP102 with FLAG-tagged versions of the wild-type A2A receptor (lane 1), the A2A receptor-383RVRAA387, a version with a mutated DVELL motif (lane 2), and the truncated A2A receptor(1–360) (lane 3) or cotransfected with an empty plasmid (lane 4) were resolved electrophoretically, transferred to nitrocellulose, and probed with the monoclonal antibody directed against the A2A receptor (D).