FIGURE 1.
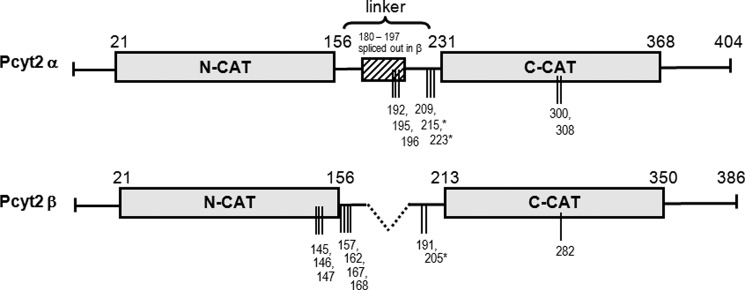
Domain structure of Pcyt2α and -β and phosphorylation sites identified in this study. Pcyt2β splicing results in the deletion of residues 180–197 from the central linker segment. The layout of the catalytic domains (N-cat and C-cat) is scaled proportionately, as is the linker segment. Phosphorylation sites identified by mass spectroscopy are represented by vertical lines, and * indicates phosphorylations at PKC consensus sites. Pcyt2α is longer and has 404 residues. Pcyt2β is the product of internal splicing of central 18-mer α-specific motif and it has 386 residues.
