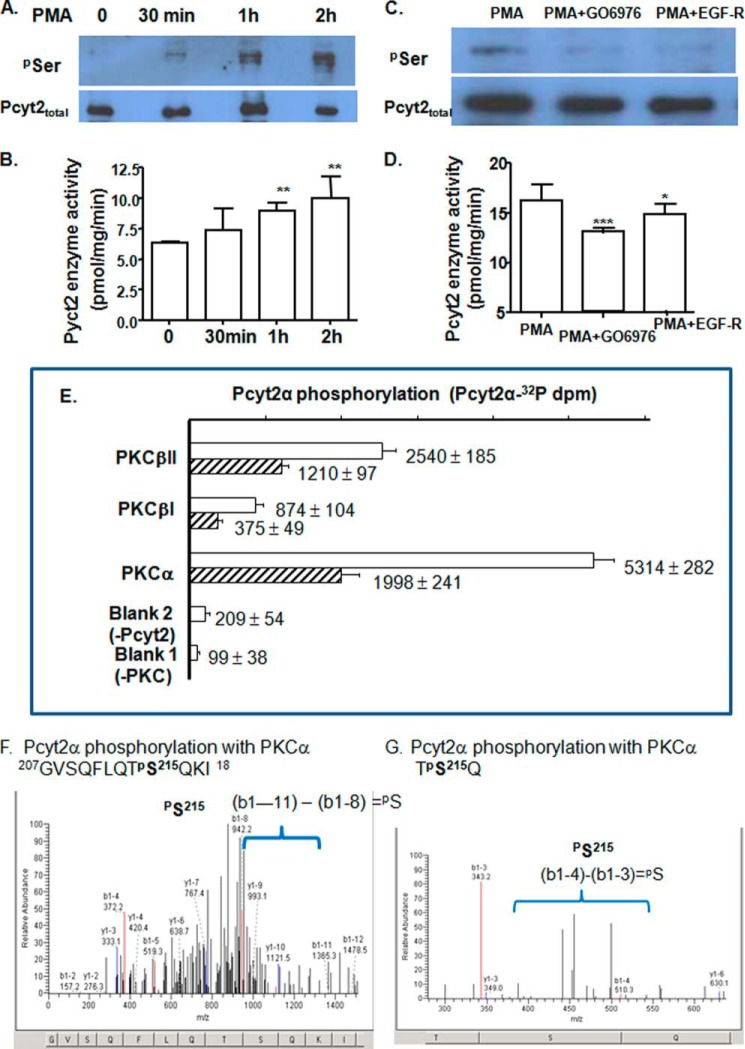
FIGURE 4.
In vivo and in vitro regulation of Pcyt2 with PKC. Phosphorylation (A) and catalytic activity (B) of endogenous Pcyt2 were analyzed after a short term treatment (0.5, 1, and 2 h) of serum-starved MCF-7 cells with the PKC activator PMA. PMA (25 nm)-induced Pcyt2 phosphorylation (C) and catalytic activity (D) were measured after 0.5 h of treatment with specific PKC inhibitors Gö6976 (50 nm) and EGF-R (50 nm); Pcyt2 immunoprecipitation and activity assays were performed as described under “Experimental Procedures.” Endogenous Pcyt2 was immunoprecipitated with Pcyt2total antibody, and phosphorylated Pcyt2 was identified by Ser(P) antibody. Pcyt2 enzyme activity was determined under the same conditions using [14C]phosphoethanolamine as a substrate. Pcyt2 activity is in picomoles/mg/protein ± S.D. at p < 0.05 (*), p <0.01 (**), and p <0.001 (***). Results are from three independent experiments. E, in vitro transcribed-translated Pcyt2α (produced untagged and devoid of modifications by phosphorylation) was phosphorylated with [32P]ATP and PKCα, PKC βI, or PKCβII. The incorporation of [32P]ATP was measured in triplicate (dpm ± S.D.) at two Pcyt2α concentrations (0.1 μg (hatched bars) and 0.2 μg (open bars). The 32P activity incorporated into Pcyt2α was compared with assays without Pcyt2 or PKC added. F and G, overexpressed V5/His-tagged Pcyt2α was purified from serum-treated MCF-7 cells (conditions established to produce minimal phosphorylation) and was subjected to the PKCα assays as above, however, using unlabeled ATP. The phosphorylated peptides were separated and characterized by MALDI-Qq-TOF tandem mass spectroscopy. Shown are the two m/z fragmentations for PKCα-treated Pcyt2α. Both patterns identify Pcyt2α phosphorylation at Ser-215.