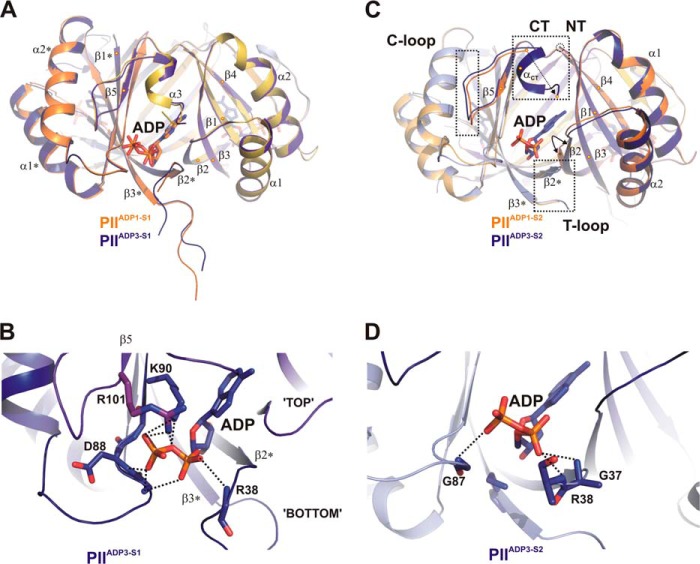
FIGURE 3.
Transition between structures PIIADP1 and PIIADP3 and differential binding mode of ADP. A, superposition of the PIIADP1 (orange and yellow) and PIIADP3 (dark blue and magenta) structures shown as schematics with side views of the S1 effector binding sites. Secondary structure elements are marked with α1-α2 and β1-β4 (with * for the second molecule). The ADP cofactors are represented by stick structures and color-coded according to the protein backbone (orange and blue). B, close-up of the S1 site of PIIADP3 highlighting residues (labeled and represented by stick structures) involved in ADP binding. C, comparison of the structures of the S2 binding site of PIIADP1 and PIIADP3. The color codes are the same as for A, but additional subunits are light orange (PIIADP1) and light blue (PIIADP3). Significant structural changes in B-loop, T-loop, and C termini induced by ADP binding are enclosed in dotted rectangles. D, close-up of the S2 site of PIIADP3. ADP is bound only via main-chain interactions to residues of the B-loop and T-loop.