FIGURE 11.
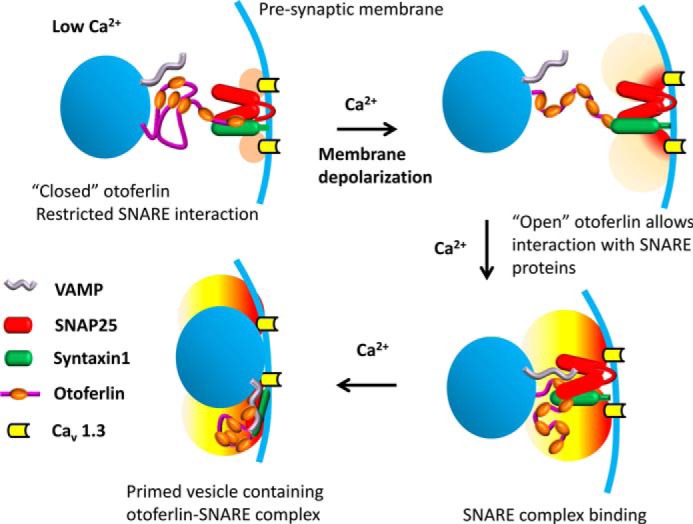
Model illustrating calcium regulation of otoferlin/SNARE interaction in the hair cell. It has been shown that otoferlin C2 domains interact with each other maximally in the absence of calcium, and when calcium levels increase beyond 10 μm, the interaction is reduced significantly (Fig. 10) except for domains C2A and C2B, which remain unaffected (supplemental Fig. 3). Based upon these observations, it is proposed that otoferlin may undergo a conformational shift from closed (top left) to open (top right) during a presynaptic increase in calcium. Closed otoferlin may be restricted in its interaction with other proteins, especially the C2C, C2D, C2E, and C2F interactions with t-SNARE proteins. As calcium increases, the opened otoferlin interacts with t-SNAREs (bottom right), facilitating vesicle-presynaptic membrane fusion (bottom left) (vesicles, blue spheres; presynaptic membrane, blue curve). The “halo” near the calcium channels indicates the calcium level with light orange suggesting low calcium (upper left), light red showing higher calcium entering (upper right), and yellow indicating the development of an intermediate calcium level attained with continuing calcium entry (lower diagrams). Consistent with this model, the predepolarization calcium concentration at the inner hair cell ribbon synapse is estimated to be ∼10 μm or less (56). There is little C2F interaction with the other C2 domains observed above 10 μm calcium (Fig. 10F). On the other hand, C2D, C2E, and C2F interactions with syntaxin-1 t-SNARE motif are stronger above 10 μm calcium, e.g. at 20 μm calcium (Table 2; KD = ∼10−9). The otoferlin/SNARE interaction at low calcium concentrations may correspond to baseline levels of exocytosis. VAMP, vesicle-associated membrane protein.
