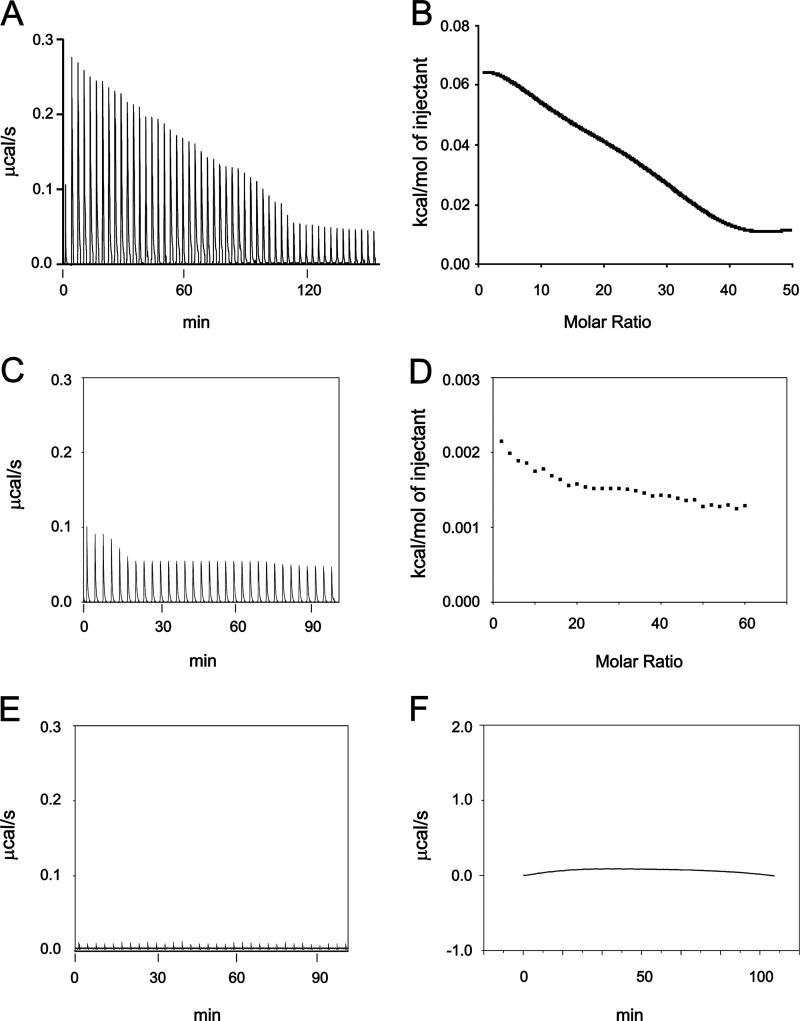
FIGURE 4.
ITC measurement of otoferlin C2F domain calcium binding. Affinity-purified wild-type GST-C2F otoferlin protein and a fusion protein representing an otoferlin pachanga mutant (see text) were cleaved with PreScission protease (GE Healthcare) to remove the GST tag. Individual 5-μl volumes of a 5–10 mm CaCl2 solution prepared in ITC buffer were incrementally injected into an ITC chamber (Microcal ITC200) containing a 50–100 μm concentration of the purified C2F domain solution at 25 °C, and the heat change was recorded. Negative controls were either C2F samples injected with ITC buffer alone or ITC buffer injected with 10 mm CaCl2. A, raw data obtained in the titration of C2F domain by calcium. The CaCl2 solution was injected 50 times, and the response was recorded. The binding reaction was endothermic. Fitted data for the integrated heat change on injection of CaCl2 were plotted after subtraction from control values. B, curve fitting for A performed with Microcal Origin software using a two-calcium binding model. One main binding site was predicted with a KD of 267 μm. A second predicted site was in the mm range, which likely reflects nonspecific heat changes. C and D, raw data and corresponding curve fitting, respectively, obtained for the C2F pachanga point mutation (Asp to Gly). Experimental conditions were identical to those for the wild-type C2F. Data indicate a very small endothermic response with little reduction in the titration, suggesting either no binding or low affinity binding. Minimal changes in integrated heat did not allow determination of affinity values. E and F, raw data for control experiments. E, increments of ITC buffer alone (no added CaCl2) added to a 50 μm solution of wild-type C2F. No heat response was associated with the injections. F, ITC buffer alone incrementally injected with ITC buffer containing 10 mm CaCl2. A negligible endothermic response was observed for each injection, probably indicating the heat of calcium dilution.