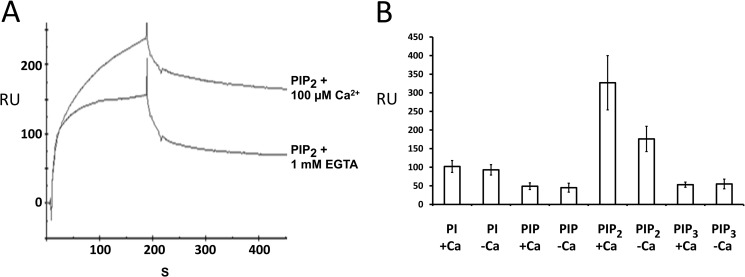
FIGURE 5.
Otoferlin C2 domain interacts with phosphatidylinositols. A, surface plasmon resonance analysis of otoferlin C2F interaction with PIP2. Purified C2F domain was immobilized on a CM5 sensor chip via an amine coupling reaction (11). 100 nm PIP2 (analyte) was diluted in HBS binding buffer containing no added Ca2+, and the solution was brought to 100 μm Ca2+ before injection. For binding assays lacking Ca2+, the analyte was adjusted to 1 mm EGTA. Samples were injected at a flow rate of 20 μl/min. Flow cells blocked with ethanolamine served as reference cells. Each sample was analyzed in five replicas, and all experiments were performed at least three times. There was approximately a 40% increase in binding for PIP2 in the presence of Ca2+. B, a 100 nm concentration of each of phosphatidylinositol (PI), PIP, PIP2, and PIP3 was analyzed on a C2F-immobilized sensor chip, and the maximum binding response at the end of the association phase (n = 5) was averaged. Error bars denote S.E. Among the different phosphoinositides and phosphatidylinositol, PIP2 showed the most robust binding to the C2F domain followed by phosphatidylinositol, PIP3, and PIP. PIP2 also showed a significantly higher response than the other phosphoinositols in the presence versus absence of 100 μm calcium. Phosphatidylinositol, PIP, and PIP3 did not show any remarkable increase in C2F binding upon addition of calcium. RU, response units.