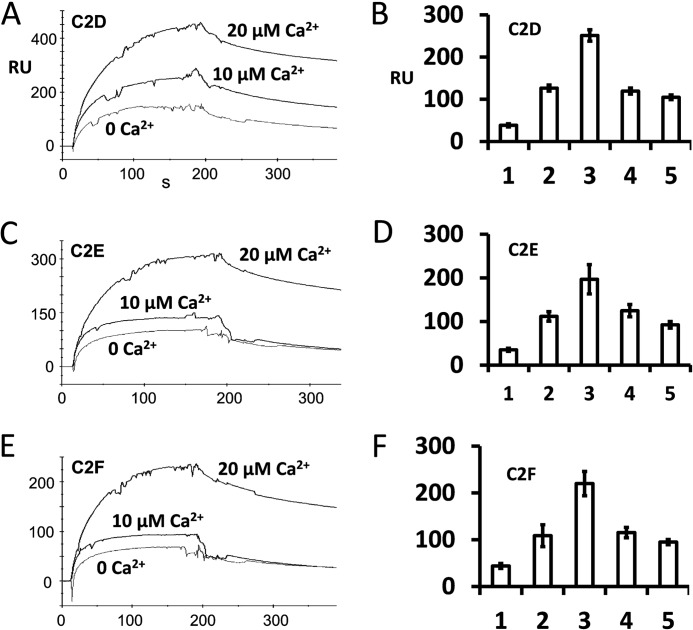
FIGURE 9.
Calcium-dependent interaction of otoferlin C2 domains with the syntaxin-1 SNARE motif determined by surface plasmon resonance. A, otoferlin C2D domain (ligand) was immobilized on a CM5 sensor chip (Biacore) by the amine coupling reaction (11). Purified syntaxin-1 SNARE fusion protein was diluted in HBS buffer (analyte), and different amounts of calcium were added (indicated in A, C, and E). Maximum SPR response at the end of the injection (i.e. the end of the association phase) obtained from each binding curve shows the relative binding (ligand binding subtracted from reference binding). Maximum interaction of C2D with the SNARE motif protein was observed at 20 μm Ca2+ (top trace). Binding was reduced when 1 mm EGTA was added to the binding buffer (0 Ca2+). B, binding responses expressed graphically for C2D with the SNARE motif. Bars 1, 2, 3, 4, and 5 represent average maximum binding ±S.E. (error bars) for 0, 10, 20, 50, and 100 μm Ca2+, respectively. Three independent values were averaged for each condition. Bars 1–3 correspond to the plots shown in A. There was a significant increase in binding (∼6.6-fold) at 20 μm Ca2+ compared with 0 μm Ca2+. However, a further increase in calcium did not increase the binding; rather, a decline in binding was observed. C, SPR binding curves in a format similar to that shown in A for purified C2E domain as ligand and purified SNARE fusion protein as analyte. Again, maximum binding occurred when Ca2+ reached 20 μm. D, averaged maximum binding responses for C2E with the SNARE motif. The format is similar to that for B. There was a 5.6-fold increase in binding at 20 μm Ca2+ (bar 3) versus 0 μm Ca2+. E, similar SPR binding curves for C2F as ligand and SNARE fusion protein as analyte. F, similar averaged binding responses obtained for the C2F domain with the SNARE motif. A ∼5-fold increase in binding at 20 μm calcium versus 0 μm calcium was observed.