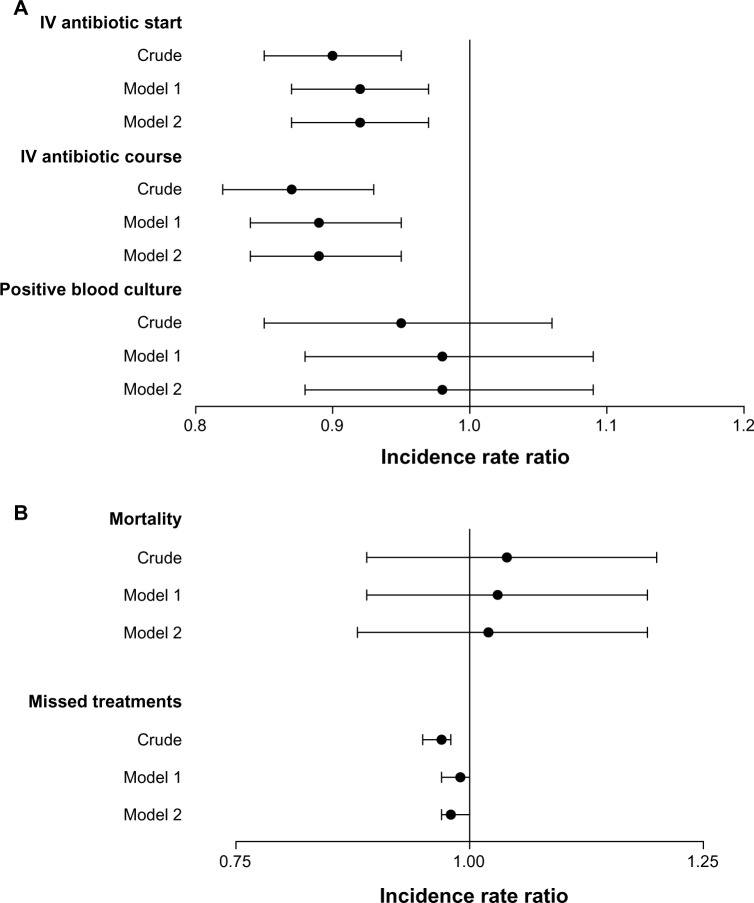
Figure 1.
Associations between Tego use and catheter-related bloodstream infection (CRBSI), mortality, and missed dialysis treatments. Incidence-rate ratios (IRRs) with 95% confidence intervals are shown for (A) metrics of CRBSI and (B) mortality and missed dialysis treatments. IRRs for Tego versus controls are shown unadjusted (crude); adjusted for age, race, vintage, and etiology of ESRD (model 1); model 2 adjusted for all variables (age, sex, race, vintage, primary cause of ESRD, diabetes, heart failure, ischemic disease, cerebrovascular disease, cardiac dysrythmia, COPD, liver disease, malignancy, and Charlson comorbidity index).
Note: Tego needlefree connector is manufactured by ICU Medical, Inc., San Clemente, CA, USA.
Abbreviations: IV, intravenous; ESRD, end-stage renal disease.