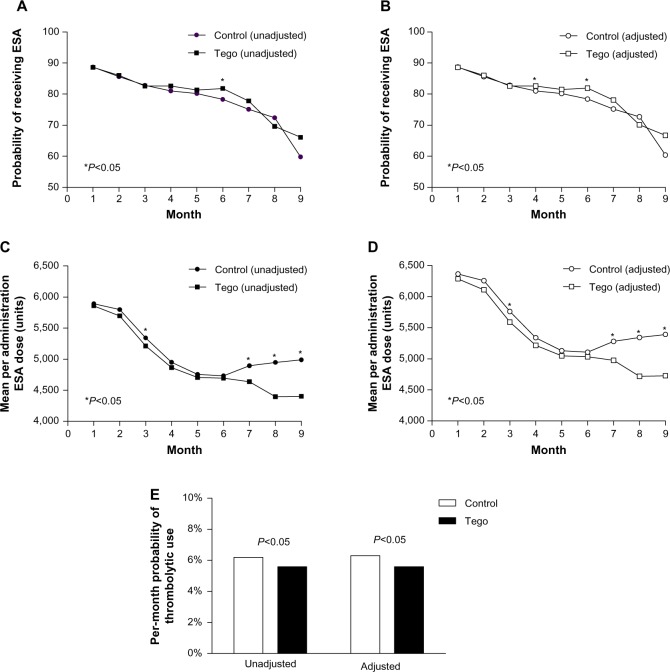
Figure 2.
(A–E) Medication utilization for Tego and control cohorts. The probability of receiving an erythropoiesis-stimulating agent (ESA) and the per-session ESA dose among users for Tego and control patients in each month of the study are shown. (A and C) Unadjusted estimates; (B and D) estimates adjusted for age, race, vintage, and etiology of end-stage renal disease (model 1). Unadjusted and adjusted (model 1) estimates of the per-month probability of thrombolytic use over the entire study period are shown in panel E.
Notes: *Months in which the difference between Tego and control groups was statistically significant (P<0.05). Tego needlefree connector is manufactured by ICU Medical, Inc., San Clemente, CA, USA.