Abstract
Peer- and family-based group therapies have been used as separate interventions to improve adjustment and self-management among youth with Type 1 diabetes mellitus. This study replicates a treatment protocol that combined these two types of diabetes management groups, while also using a wait-list control design methodology within an outpatient mental health clinic setting. General psychosocial and diabetes-related variables were assessed at baseline, immediately posttreatment, and 4 months posttreatment. Youths' medical information, including metabolic control values, was extracted from medical charts for the 6 months prior to baseline and 6 months after treatment ended. At 4 months posttreatment, parents and youth reported increased parent responsibility, and parents reported improved youth diabetes-specific quality of life. Although there were no statistically significant changes in hemoglobin A1c values and health care utilization frequency from 6 months prior to and 6 months posttreatment, other psychosocial changes (i.e., increases in parent responsibility and diabetes-specific quality of life) were documented. Therefore, this treatment was found to be a promising intervention for use in an outpatient clinical setting to aid in improving the psychosocial functioning of youth with Type 1 diabetes mellitus.
Keywords: Type 1 diabetes, group interventions, adolescents, parents, peers
Group interventions for specific pediatric populations exist and have been shown to be beneficial, but researchers suggest that much work is still needed to establish their effectiveness (Plante et al., 2001). Two types of therapy intervention modalities, peer group and family based, have often been used with youth who have Type 1 diabetes mellitus (T1DM). Various peer group interventions for adolescents with T1DM have focused on a wide variety of topics, such as providing peer support and diabetes knowledge as well as developing problem-solving, coping, and stress management skills (Anderson, Wolf, Burkhart, Cornell, & Bacon, 1989; Boardway, Delamater, Tomakowsky, & Gutai, 1999; Boland, Grey, Oesterle, Frederickson, & Tamborlane, 1999; Greco, Pendley, McDonell, & Reeves, 2001; Grey et al., 1998; Kaplan, Chadwick, & Schimmel, 1985; Mendez & Belendez, 1997). These studies demonstrated improved short-term hemoglobin A1c (HbA1c) levels, diabetes-related stress, and quality of life (QOL) as well as improved adolescent social interaction about diabetes.
Family-based interventions, such as Multi-systemic Therapy (MST; Ellis et al., 2005) and Behavioral Family Systems Therapy (BFST; Wysocki et al., 2000), have also been shown to be effective in improving diabetes management in adolescents. Ellis et al. (2005) demonstrated that intensive, individual MST family-based interventions at home improved frequency of blood glucose testing and metabolic control as well as decreased inpatient admissions among patients with T1DM, who chronically evidence poor diabetes control. Wysocki et al. (2007) found that family-based interventions using BFST with adolescents showed improvement in parent–adolescent relationships and HbA1c levels as well as reduced diabetes-specific conflict compared with a randomized educational support or a standard care group.
A review of both the group-based and family-based intervention research shows promise for positive benefits for adolescents with T1DM; however, clinical trials are still needed to establish these interventions as being effective and efficacious treatments (Plante et al., 2001). A number of recommendations have been made about how to strengthen the body of research for evaluating group therapy interventions in pediatric populations. Specifically, more longitudinal research that incorporates a variety of measurement approaches and outcome measures is warranted to determine potential causal relationships among psychosocial factors and the management of medical conditions (Delamater et al., 2001). It is necessary to examine the impact of group and family treatments in outpatient settings to increase the external validity of the findings (Plante et al., 2001). In addition to establishing the efficacy and then the effectiveness of an intervention, researchers need to assess the cost savings of an intervention (Stark et al., 1996). It is estimated that a hospital admission for diabetes ketoacidosis in an individual with T1DM on an insulin pump can be as expensive as $13,000 per episode (Garg et al., 2004). Thus, decreasing the frequency of even one hospitalization for a patient through an outpatient intervention has the potential to impact health care costs. Longitudinal designs that utilize random assignment of participants to waitlist control (WLC) groups in an outpatient setting will enhance the literature on group treatment.
Opipari-Arrigan and colleagues (2005) developed the Kicking in Diabetes Support (K.I. D.S.) Project, which provides both peer group and family-based interventions to adolescents with T1DM and their parents. The present study extends the evidence base for the K.I.D.S. Project by implementing the same treatment protocol in an outpatient mental health setting with a clinical population. Although participants in the original study, which evaluated the K.I.D.S. Project, were recruited exclusively for a grant-funded, paid treatment intervention, the participants in the present study were patients from the diabetes clinic referred for treatment of psychosocial issues to an outpatient mental health center. All patients with T1DM in the specified age range (e.g., 13–17 years old) were offered the clinical group intervention in place of individual therapy. Thus, the present study population represents a more heterogeneous and “real world” sample than other studies that rely on recruitment solely for research study purposes. Conducting this intervention for a clinical population in the context of a WLC experimental design will help address the gap in existing research by providing a preliminary efficacy study for this intervention (Chambless & Ollendick, 2001). Specifically, the present study seeks to establish the feasibility of providing the K.I.D.S. Project intervention in an outpatient clinical setting and demonstrate that this clinical intervention is acceptable, available, and adaptable for providers and trainees to utilize (Flay, 1986).
The previous research findings from Opipari-Arrigan et al. (2005) guided the selection of the measures for the present study. Participants in the original K.I.D.S. Project evaluation completed measures of general and diabetes-specific QOL, general psychosocial functioning, parental distress, regimen adherence, diabetes responsibility, diabetes conflict, diabetes knowledge, adolescent adjustment to diabetes, and diabetes support. There were improvements in youth's responsibility and general and diabetes-specific QOL as well as evidence of stable glycemic control levels over the 12-month follow-up period. In addition, previous results by Kaugars, Kichler, and Alemzadeh (2011) were used to provide a rationale for the inclusion of a measure of readiness to change the balance of responsibility of diabetes care from parent to youth. Each item on that measure asks respondents to choose the statements that represent their readiness to change the balance of responsibility for diabetes cares, within the framework of the stages of precontemplation, contemplation, preparation, action, and maintenance. Kaugars et al. (2011) found that greater parental readiness to change the balance of responsibility of diabetes care from parent to youth was related to more youth diabetes responsibility and less general parental stress. In order to assess diabetes adherence changes over time, a well-established measure of self-care adherence was utilized in the present study (La Greca, Swales, Klemp, & Madigan, 1988).
The specific aim of this project was to implement the K.I.D.S. Project intervention to determine the impact of this treatment on improving psychosocial adjustment and diabetes management among adolescents with T1DM and their parents using a WLC design methodology in an outpatient clinical mental health setting. The proposed within-group hypotheses were as follows: (1) adolescent and parent general psycho-social and diabetes-related improvements from baseline to posttreatment as well as maintenance of these changes at 4 months posttreatment and (2) adolescent health care utilization and metabolic control improvements from 6 months prior to baseline to 6 months posttreatment. The proposed between-groups hypotheses were as follows: (1) no differences between the treatment and WLC groups at the baseline assessment (i.e., prior to randomization) on measures of psychosocial functioning, diabetes management, health care utilization, or metabolic control and (2) improved scores on measures of psychosocial functioning, diabetes management, health care utilization, and metabolic control for participants in the treatment group at their immediate posttreatment assessment compared with participants in the WLC group at their second pretreatment assessment (i.e., before starting the intervention).
Method
Participants
Participants in the present study were 30 adolescents with T1DM for at least 6 months between 13 and 17 years of age, who were patients of a diabetes clinic in a large, midwestern hospital and their parents. Adolescents with T1DM were included if they had other chronic medical diseases, such as celiac disease, or coexisting mental health disorders if they were on stable psychotropic medications (i.e., dose stable for at least 3 months). Potential participants were excluded if (a) they had a coexisting diagnosis of mental retardation, pervasive developmental disorder, substance abuse, eating disorder, or psychosis or other acute psychiatric or medical needs, such as suicidality, or (b) they were not fluent in the English language.
Design and Procedure
Adolescent participants and their parents were recruited in three waves: First wave: treatment group (n = 6) and WLC group (n = 6); Second wave: treatment group (n = 5) and WLC group (n = 6); and Third wave: treatment group (n = 4) and a WLC group (n = 4). One participant in the third wave treatment group participated in the clinical aspects of the group, but did not meet inclusion criteria for the research portion of the study (i.e., the patient had Mature Onset Diabetes of the Young and not T1DM). Therefore, the participant's data were not included in present analyses. Participants were recruited by one of the following methods: advertisement in the diabetes clinic's mailings, postings of the advertisement flyer in the clinic waiting room, distribution of a flyer describing the group during a clinical appointment or class, or referral to the outpatient mental health clinic for psychological services to address concerns regarding diabetes adjustment and coping.
Families contacted the outpatient mental health clinic, which is separate from the endocrinology clinic, to be screened for clinical appropriateness of their participation in the group therapy. An insurance verification was completed to determine insurance coverage for participating in the group. Participants were outpatient mental health clinic patients and were responsible for all costs associated with care, including group therapy charges. If a family was not eligible or declined to participate in the group therapy, they were referred for individual therapy. For those participants who did qualify and verbally agreed to participate in the group intervention, plans were made for the family to participate in an intake session with a licensed psychologist. After the initial intake visit, if the family remained interested in participating in this group intervention, they were randomly assigned to the treatment group (i.e., treatment offered immediately) or the WLC group (i.e., treatment offered 6 weeks after the treatment group) as a unit per the CONSORT Guidelines (Moher, Schulz, & Altman, 2001). The treatment group intervention started within 2 weeks of the intake sessions, and the WLC group intervention started 6 weeks after that.
Parental consent and adolescent assent was obtained at the initial session (baseline) before the clinical interview by one of the two licensed psychologists leading the groups. Parents and adolescents then both completed standardized measures of psychosocial and diabetes functioning (i.e., general and diabetes-specific QOL, adolescent emotional and behavioral functioning, adherence, readiness to change the balance of responsibility, and responsibility allocation). Parents also completed demographic, parent stress, and health-related family impact measures. For both the treatment and the WLC groups, these measures were given again at posttreatment and 4 months after baseline. For the WLC group, these measures were given one additional time at the initiation of their intervention (pretreatment). The questionnaires took approximately 30 min for the adolescents and 45 min for the parents to complete at each assessment time point.
In addition to the self-report measures, each participant's medical record was reviewed for the 6 months prior to and the 6 months after the baseline visit. For the participants in the WLC group, their medical record review also included the WLC time period (i.e., the time between the baseline assessment and the 6-week delay in treatment initiation) in their baseline assessment. The medical record review yielded the following information about the participating adolescents: height, weight, Tanner staging scores, number of hospital admissions and emergency room visits related to T1DM, and HbA1c levels recorded from outpatient diabetes clinic visit notes during the duration of the study. HbA1c was determined by the Bayer DCA (Bayer Diagnostics Inc., Tarrytown, NY) 2000 instrument (nondiabetes range of 4.5% to 5.7%). Health care utilization was defined as the number of unique hospitalizations and/or emergency room visits related to T1DM that the participant had during the study time frame. The duration and type of diabetes reported was also verified during the medical record review. Institutional Review Board approval was obtained from the participating institutions.
Overview of Intervention Protocol
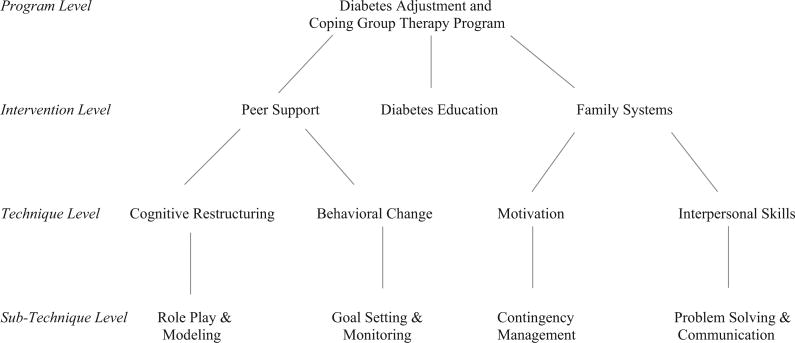
The K.I.D.S. Project intervention is a synthesis of treatment strategies from the diabetes education, behavior therapy, and family therapy literature. The diabetes education literature provides both the necessary clinical information for effective T1DM management, as well as the approach for presenting the clinical, behavioral, and psychosocial information in an integrated format that empowers the patient and parent to become informed decision makers. The behavior therapy literature provides techniques to engage adolescents in the behavior change process and strategies for parents to implement and encourage positive health care choices in their adolescent. The family therapy literature provides techniques in working within the family system to change communication patterns, decrease interpersonal conflict, and build the framework that the family is a “team” working together (see Figure 1).
Figure 1.
Group Intervention Program Tree Diagram.
This intervention is summarized in a semi-structured manual format for both adolescents and parents, where session goals, topics, information, and activities are all prepared for the leaders to use as a reference to help provide a framework for each session (Opipari-Arrigan et al., 2005). The group therapy sessions all have similar topic areas for both the parents and the adolescents to address during the six intervention sessions; topics include consideration of developmental aspects to diabetes management during adolescence, parent involvement and communication, goal setting and problem solving, behavioral contingency and contracting, and school and peer issues. The parents receive a binder of diabetes education materials and initial “survival guide” guidelines for managing T1DM. These materials also include informational and interactive worksheets for behavioral management and are utilized during the group sessions.
Structure of Group Sessions
Group sessions are conducted for adolescents and parents separately for the first portion of each session and then for all the families together for the second portion of each session. The parent and adolescent sessions are each led by a licensed psychologist and the adolescent session has a psychology graduate student trainee as a cotherapist. These group leaders were consistent throughout the intervention for the families. The diabetes education and behavioral intervention information presented to both the adolescent and the parent groups are guided by participants' individual concerns and questions, and diabetes-specific activities are used to reinforce topics discussed each week (see Figure 1). The activities in the adolescent group focus on building rapport among group members, exploring shared diabetes experiences, enhancing diabetes knowledge, increasing efficiency at carbohydrate counting, practicing skills with an experiential exercise activity and blood glucose monitoring, role playing and modeling of typical social and school-based scenarios, and fostering parent–child collaboration and teamwork. These activities are then followed-up with guided group discussion and support among the peers to facilitate behavior change.
Following the separate parent and adolescent portion of each group (approximately 30–45 min), the parents and adolescents come back together in parent–adolescent units to work on individual family goals for the last portion (20–30 min) of the group session. The family portion of the sessions focuses on practicing negotiation skills for goal setting and problem solving in parent–adolescent dyads in vivo. Diabetes goals are specific to each family and are based on the issues identified by both the adolescent and the parent during the separate sessions. The group leaders allow parents and adolescents to engage in family negotiation tasks as independently as possible and may provide supportive coaching as needed throughout the course of the intervention. The approach allows group therapy leaders to provide the basic foundation of behavioral and family systems strategies, while tailoring the content of the material on individual goals based on the participants' needs (see Figure 1).
Measures
General psychosocial functioning
General demographic and family history form
This questionnaire assesses general demographic information, family constellation, diabetes diagnosis duration, and family history of other medical and psychological conditions (Kichler & Crowther, 2001). The demographic form was completed by parents at baseline and updated at follow-up assessments, as needed.
The Brief Symptom Inventory (BSI-18)
The BSI-18 is an abbreviated 18-item version of the original BSI (53 items) that assesses three dimensions of adult psychological distress (i.e., somatization, depression, and anxiety) (Derogatis, 1993). Respondents rate their perceived severity of symptoms experienced during the previous 7 days on a 5-point scale ranging from 0 (not at all) to 4 (extremely). According to Derogatis (1993), the BSI has adequate internal consistency (rs = 0.71–0.85) and test–retest reliability (rs = 0.68–0.91), and the BSI-18 is correlated with the Symptom Checklist-90-Revised (rs > 0.90). Parents completed this measure at baseline and at all follow-up assessments. Parents' Global Severity Index score, which assesses overall distress, was used in the present analyses (baseline α = .88).
Behavioral Assessment Scales for Children (BASC-2)
The parent form (Parent Rating Scale [PRS]) is a comprehensive measure of a child's adaptive and problem behaviors in community and home settings (Reynolds & Kamphaus, 2004). The PRS uses a four-choice Likert-type response format where higher scores indicate more problem behaviors and yields composite scores of Externalizing Problems, Internalizing Problems, Other Problems, Adaptive Skills, and a Behavioral Symptoms Index score. Internal consistency for PRS composite scores ranges from 0.88 to 0.93 and test–retest reliability from 0.89 to 0.94 (Reynolds & Kamphaus, 2004). The youth form (Self-Report of Personality [SRP]) is a personality inventory consisting of statements that are responded to as True or False and Likert-type responses. Composite scores include School Problems, Internalizing Problems, Inattention/Hyper-activity, Personal Adjustment, and an overall Emotional Symptoms Index. Internal consistency for SRP composite scores ranges from 0.87 to 0.95 and test–retest reliability from 0.87 to 0.96 (Reynolds & Kamphaus, 2004). The parents completed the PRS form, and the adolescents completed the SRP at baseline and all follow-up assessments. The parent-report Behavioral Symptoms Index (BSI) scores and the adolescent-report Emotional Symptoms Index (ESI) scores were utilized for this study.
Pediatric Quality of Life Inventory–Generic Core Scales (PedsQL)–Short Form
The PedsQL 4.0 Generic Core Scale Short Form is a 15-item inventory that assesses health-related QOL in youth ages 2 to 18 in four domains: Physical Functioning, Emotional Functioning, Social Functioning, and School Functioning (Varni, Seid, & Kurtin, 2001). At baseline and follow-up assessments, parents and adolescents provided ratings on a 5-point Likert scale, where higher scores reflect better QOL. In addition, a Total score and two summary scores (i.e., Psychosocial Health and Physical Health) can be calculated. Internal consistency is good with alphas of 0.88 for the child report and 0.90 for parents' reports (Varni et al., 2001). The PedsQL Psychosocial Health summary scores for both the parents and the adolescents were utilized for this study (baseline αs = 0.89 and 0.84, respectively).
The Pediatric Quality of Life Family Impact Module (PedsQL FI)
The PedsQL FI is a parent-report measure with 36 items rated on a 5-point Likert scale, where higher scores indicate better parent and/or family functioning (Varni, Sherman, Burwinkle, Dickinson, & Dixon, 2004). There are eight dimensions of parent and family functioning: Parent Physical Functioning, Parent Emotional Functioning, Parent Social Functioning, Parent Cognitive Functioning, Communication, Worry, Daily Activities, and Family Relationships. A Total score and two summary scores (Parent Health Related Quality of Life [HRQL] and Family Functioning) can be computed. Parents completed this measure at baseline and all follow-up assessments. The PedsQL FI Total score was utilized for the present study (baseline α = 0.97).
Diabetes-specific functioning
Readiness to Change the Balance of Responsibility Scale (RCBRS)
The RCBRS youth version assesses how prepared the adolescent is to take direct responsibility for a specific diabetes-related behavior while a parent supervises (Kaugars et al., 2011). Items are rated on a 5-point Likert scale, where higher scores represent more readiness to change. The parent version includes additional questions about factors relevant to the transfer of responsibility. Acceptable internal consistencies for the mean scores have been demonstrated (maternal α = 0.74, paternal α = 0.64, youth α = 0.76; Kaugars et al., 2011). The parents and youth filled out these measures at baseline and follow-up assessments. A mean score of the 12 items (parent) and the seven items (youth) was used in the present study (baseline αs = 0.89 and 0.57, respectively).
Self-Care Inventory (SCI)
This self-report questionnaire measures adherence to the diabetes regimen across a series of self-care activities (e.g., glucose testing and attending appointments) (La Greca et al., 1988). Items are rated on a 5-point Likert scale, where higher scores indicate better adherence to diabetes treatment recommendations. Adequate internal consistency (α = 0.87) has been reported (La Greca et al., 1988). Both parents and adolescents completed this measure at baseline and follow-up assessments. An item-average score of the SCI by the parents and adolescents was utilized in this study (baseline αs = 0.82 for both).
Diabetes Family Responsibility Questionnaire (DFRQ)
The DFRQ is a 17-item self-report instrument designed to measure family allocation of diabetes management tasks (Anderson, Auslander, Jung, Miller, & Santiago, 1990, Anderson et al., 2002). Items are rated on a 3-point Likert scale, where higher scores indicate the child is taking more responsibility for a task than the parent. For each situation or task, respondents are asked to indicate whether the parent or child initiates responsibility almost all of the time or whether the parent and child share responsibility. Responsibilities are reflected in three domains: General Health Maintenance, Regimen Tasks, and Social Presentation. The three subscales have acceptable internal consistency (αs = 0.69 to 0.79) and an alpha of 0.85 for the Total scale (Anderson et al., 2002). Parents and adolescents both completed this measure at baseline and follow-up assessments. The average DFRQ total score was calculated in the present study for both parents and adolescents (baseline αs = 0.67 and 0.51, respectively).
Pediatric Quality of Life Inventory–Diabetes Module (PedsQL Diabetes)
The PedsQL 3.0 Diabetes Module consists of 28 items that assesses five summary score scales: Diabetes-Specific health, Treatment Barriers, Treatment Adherence, Worry, and Communication (Varni et al., 2003). Respondents rate on a 5-point Likert scale, where higher scores reflect better diabetes-specific QOL. The measure has acceptable internal consistency for most of the summary score scales (average αs = 0.71 for child/adolescent and 0.77 for parent reports), including the strongest alphas for the Diabetes-Specific Health summary score (α = 0.81 for children/adolescent and parent reports; Varni et al., 2003). The PedsQL Diabetes measure was completed by both parents and adolescents at baseline and follow-up assessments. The Diabetes-Specific Health summary score was calculated for the present study for both parents and adolescents (baseline αs = 0.70 and 0.85, respectively).
Statistical Analyses
All analyses were conducted with the Statistical Package for the Social Sciences, Version 19.0 (SPSS, Inc.). Probability levels of p < 0.05 were used as a cutoff for statistical significance in all analyses. As this study is a pilot investigation, it was determined that in order to find a large effect size of the outcome variables, a sample of 26 participants was needed as a rule-of-thumb power estimate (Cohen, 1992). Descriptive and correlational analyses were conducted with participant baseline characteristics. For the parental data, a primary caregiver was identified as the parent/caregiver who participated the most in the group intervention with an adolescent (maternal caregivers: n = 28; paternal caregivers: n = 2).
In order to compare the within-group study variable values across time for multiple measures, a repeated-measures MANOVA was used to compare psychosocial and diabetes-related outcome variables between baseline, posttreatment, and 4 month posttreatment follow-up for both parent and adolescent responses separately. Bonferroni's post hoc testing was applied for all significant within-group MANOVA findings to detect specific differences among the time points. In order to assess the within-group differences of health care utilization and metabolic control over time, t test comparisons of the average change in score in HbA1c and the frequency of unique hospitalization episodes were conducted from 6 months prior to baseline to 6-month posttreatment follow-up. Whenever possible, intent to treat analyses were also conducted for the whole cohort. Cohen's (1992) suggestion that effect sizes of 0.20 are small, 0.50 are medium, and 0.80 are large was utilized.
In order to compare the between-groups differences of the treatment versus WLC group at baseline (i.e., prior to randomization), MANOVAs were used to compare scores representing all the psychosocial and diabetes-related functioning constructs as well as health care utilization and metabolic control for both parent and adolescent responses separately. Then, two additional MANCOVAs were conducted to compare the between-group differences for psychosocial, diabetes-related, health care utilization, and metabolic control variables for the treatment group immediately after receiving the intervention (posttreatment) versus the WLC group participants immediately before receiving the intervention (pretreatment) for parent and adolescent responses separately, while controlling for relevant covariates.
Results
Descriptive Statistics
The adolescents' mean age at study participation was 15.17 years (SD = 1.34 years), with an average T1DM duration of 5.64 years (SD = 3.27 years). Their average age at diagnosis of T1DM was 9.54 years (SD = 3.20 years). Fifty-three percent of the adolescents were girls. The majority (76.7%; n = 23) of this sample was Caucasian, 20% (n = 6) were African American, and 3% (n = 1) were biracial African American/Caucasian. The mean body mass index standard deviation score (BMI SDS) for the sample was 0.37 (SD = 0.68).1 Adolescents' average HbA1c at baseline was 10.03% (SD = 2.06%; Range = 5.85–14.00%), and 10% of participants had been hospitalized for complications related to diabetes (e.g., diabetes ketoacidosis) during the 6 months prior to the study initiation (Range = 1.00–3.00 hospitalizations). The majority of the parents were married (83. 3%), with an additional 13.3% reporting that they were either separated or divorced, and 3.3% reported another relationship status (e.g., never married, remarried, other).
Approximately 67% of the patients received 5–6 intervention sessions (n = 20), 20% received 1–4 sessions (n = 6), and 13% received no treatment (n = 4; all of whom were in a WLC group). It is not known why these participants were lost to treatment follow-up, and they did not respond to attempts per clinic policy to contact them to attend group therapy. There were no significant correlations between the number of groups sessions attended (i.e., “dose” of intervention) and the outcome variables. Bivariate correlations of the baseline scores between the primary caregiver and adolescent on similar measures ranged from r = 0.09 to 0.53; however, there were only statistically significant relationships between reporters on the SCI and the PedsQL Generic forms. Baseline characteristics and intent to treat analyses for medical/metabolic control variables were evaluated for the whole cohort (n = 30), but only the data from the participants who received at least one session of the intervention were included in the within- and between-groups analyses (n = 26).
Comparisons between the treatment and WLC groups on baseline demographic variables (e.g., age, length of time since diabetes diagnosis, BMI, or pubertal development) did not yield any significant differences. There were two significant differences at baseline for the parent ratings of the PedsQL FI and the youth ratings of the SCI, t(28) = 2.71, p < 0.05, and t(28 = −2.80, p < 0.01, respectively. Participants in the treatment groups had higher parent-reported QOL family impact ratings and lower adolescent-reported diabetes adherence ratings compared with the ratings of the WLC groups prior to randomization. Therefore, these variables were used as covariates in the between-group MANCOVAs for both the parent and adolescent analyses.
Within-Group Differences Across Time
The within-group comparisons found that some of the psychosocial and diabetes-related variables varied across time from baseline, post-treatment, and 4-month posttreatment follow-up (See Table 1). Overall, differences in parent-reported PedQL Diabetes as well as parent and adolescent reported DFRQ scores demonstrated improvements representing small to medium effect sizes (ES range = 0.28 – 0.47), whereas parent-reported DFRQ score differences demonstrated improvements, reflecting a small effect size (ES = 0.23; Cohen, 1992). Post hoc comparisons revealed a significant difference between baseline and 4-month posttreatment follow-up for adolescent- and parent-reported DFRQ and parent-reported PedQL Diabetes scores. Parent-reported DFRQ scores were also significantly higher between posttreatment and 4-month posttreatment follow-up. Although there was an overall significant difference for the parent-reported RCBRS, the univariate post hoc analysis for the parent-reported RCBRS did not yield any significant differences across the three time points (See Table 1).
Table 1. Repeated-Measures MANOVA and Bonferroni Post Hoc Analyses for Within-Group Differences Across Time.
| Measure | Baselinea | Posttreatmenta | 4-month posttreatment follow-upa | F | p-values | Effect sizes |
|---|---|---|---|---|---|---|
| Primary caregiver | ||||||
| BASC-2 | 47.93 ± 5.25 | 48.21 ± 6.45 | 48.14 ± 7.83 | 0.04 | 0.00 | |
| BSI-18 | 42.79 ± 8.00 | 42.93 ± 8.11 | 45.07 ± 12.58 | 0.33 | 0.03 | |
| DFRQ | 1.98 ± 0.22 | 2.06 ± 0.27 | 2.17 ± 0.22*,** | 11.49 | <.01 | 0.47 |
| RCBRS | 3.97 ± 0.71 | 4.09 ± 0.66 | 4.30 ± 0.66 | 3.80 | <.05 | 0.23 |
| PedQL Generic | 81.43 ± 13.74 | 81.78 ± 13.29 | 84.29 ± 13.18 | 0.88 | 0.06 | |
| PedQL Diabetes | 64.12 ± 11.93 | 71.27 ± 11.71 | 75.97 ± 13.39* | 9.76 | <.01 | 0.43 |
| PedQL Family Impact | 71.08 ± 22.46 | 72.07 ± 17.87 | 74.45 ± 23.73 | 0.63 | 0.05 | |
| SCI | 3.40 ± 0.57 | 3.64 ± 0.46 | 3.59 ± 0.56 | 1.62 | 0.11 | |
| Youth | ||||||
| BASC-2 | 42.73 ± 6.33 | 42.53 ± 7.32 | 42.93 ± 6.50 | 0.08 | 0.00 | |
| DFRQ | 2.27 ± 0.18 | 2.35 ± 0.18 | 2.37 ± 0.15* | 5.35 | <.05 | 0.28 |
| RCBRS | 3.70 ± 0.83 | 3.91 ± 0.83 | 3.95 ± 0.62 | 1.33 | 0.09 | |
| PedQL Generic | 76.44 ± 11.07 | 74.33 ± 12.26 | 72.44 ± 13.11 | 1.65 | 0.11 | |
| PedQL Diabetes | 64.09 ± 8.29 | 63.63 ± 11.56 | 63.64 ± 13.66 | 0.01 | 0.00 | |
| SCI | 3.67 ± 0.61 | 3.87 ± 0.51 | 3.82 ± 0.49 | 2.35 | 0.14 |
Note. Boldface values indicate statistically significant findings. BASC-2 = Behavioral Assessment Scales for Children; BSI-18 = Brief Symptom Inventory; DFRQ = Diabetes Family Responsibility Questionnaire; RCBRS = Readiness to Change the Balance of Responsibility Scale; PedQL Generic = Pediatric Quality of Life Inventory–Generic Core Scales; PedQL Diabetes = Pediatric Quality of Life Inventory–Diabetes Module; PedQL Family Impact = Pediatric Quality of Life Family Impact Module; SCI = Self-Care Inventory.
Data are mean ± standard deviation.
p < 0.05, baseline versus 4-month posttreatment follow-up.
p < 0.05, posttreatment versus 4-month posttreatment follow-up.
A comparison of youths' mean health care utilization (i.e., frequency of unique diabetes-related hospitalizations/ER visits) and metabolic control (i.e., HbA1c levels) from the 6 months prior to baseline to the 6 months post-treatment was conducted. HbA1c remained stable during this time frame (baseline HbA1c = 10.04%, SD = 2.33% vs. 6-month posttreatment HbA1c = 9.74%, SD = 2.05%). The youth demonstrated a mean HbA1c change score of −0.34% (SD = 1.01%), with a range of HbA1c change scores of −3.20% to 0.80%. Similarly, health care utilization per participant remained stable (baseline hospitalizations = 0.25, SD = 0.87 vs. 6-month posttreatment hospitalizations = 0.08, SD = 0.29). An intent-to-treat analysis was also conducted for the whole cohort (n = 30), comparing mean HbA1c and health care utilization over the same time frame. There was no significant difference in HbA1c, t(26) = 1.43, p = 0.16; baseline HbA1c = 10.11%, SD = 2.09%; Range = 5.85% – 14.00% vs. 6-month posttreatment HbA1c = 9.77%, SD = 2.19%; Range = 5.90% to 14. 00%. Similarly, there was no significant difference in the frequency of health care utilization, t(29) = 0.00, p = 1.00; baseline hospitalizations = 0.20, SD = 0.67; Range = 0.00–3.00 vs. 6-month posttreatment hospitalizations = 0.20, SD = 0.76; Range = 0.00–4.00.
Between-Group Differences
Between-groups difference analyses for the treatment group at posttreatment versus the WLC group at pretreatment assessment yielded statistically significant differences on the PedsQL General and PedsQL Diabetes scores, but there were no statistically significant differences on any of the other psychosocial, diabetes-related, health care utilization, or HbA1c level variables (see Table 2). These differences reflect small-to-medium effect sizes (ES Range = 0.31 – 0.34; Cohen, 1992). Although not statistically different, the frequency of diabetes-related hospitalizations was on average 0.17 hospitalizations (SD = 0.41) per WLC group participant during the time between baseline and pretreatment assessments, whereas the treatment group participants had 0.00 hospitalizations (SD = 0.00) during the same time frame.
Table 2. Between-Group Comparison MANCOVA for Treatment Versus WLC Groups.
| Measure | Treatment group (posttreatment)a | WLC group (pretreatment)a | F | p-values | Effect sizes |
|---|---|---|---|---|---|
| Primary caregiver | |||||
| BASC-2 | 42.75 ± 9.02 | 51.50 ± 6.47 | 0.064 | 0.09 | |
| BSI-18 | 44.33 ± 7.35 | 48.92 ± 9.47 | 1.54 | 0.19 | |
| DFRQ | 2.04 ± 0.26 | 1.93 ± 0.18 | 0.15 | 0.23 | |
| RCBRS | 4.26 ± 0.55 | 3.88 ± 0.66 | 0.49 | 0.11 | |
| PedQL Generic | 73.19 ± 22.34 | 73.61 ± 18.35 | 0.48 | 0.07 | |
| PedQL Diabetes | 68.18 ± 14.11 | 60.23 ± 11.69 | 0.35 | 0.15 | |
| SCI | 3.68 ± 0.48 | 3.47 ± 0.51 | 1.96 | 0.23 | |
| Youth | |||||
| BASC-2 | 48.77 ± 13.57 | 45.08 ± 8.58 | 1.32 | 0.16 | |
| DFRQ | 2.25 ± 0.19 | 2.34 ± 0.20 | 1.76 | 0.20 | |
| RCBRS | 3.65 ± 0.84 | 4.12 ± 0.70 | 1.52 | 0.18 | |
| PedQL Generic | 66.19 ± 17.38 | 74.58 ± 9.08 | 3.14 | <0.05 | 0.31 |
| PedQL Diabetes | 58.74 ± 9.32 | 65.15 ± 9.41 | 3.66 | <0.05 | 0.34 |
Note. Boldface values indicate statistically significant findings. BASC-2 = Behavioral Assessment Scales for Children; BSI-18 = Brief Symptom Inventory; DFRQ = Diabetes Family Responsibility Questionnaire; RCBRS = Readiness to Change the Balance of Responsibility Scale; PedQL Generic = Pediatric Quality of Life Inventory–Generic Core Scales; PedQL Diabetes = Pediatric Quality of Life Inventory–Diabetes Module; SCI = Self-Care Inventory.
Data are mean ± standard deviation.
Discussion
This group therapy intervention (Opipari-Arrigan et al., 2005) provided a peer and family-based intervention to both adolescents with T1DM and their parents. The within-group comparisons over time demonstrated a significant improvement in parent-reported, diabetes-specific QOL as well as youth and parent-reported increased parental involvement in the division of diabetes responsibility. These differences were predominantly observed when comparing baseline scores to the 4-month posttreatment follow-up visit scores and not scores from baseline to immediately after treatment (post-treatment). Parent reported readiness to change the balance of responsibility for diabetes tasks scores also demonstrated a small positive increase over time, but post hoc analyses did not reveal any significant changes among the assessment points. Although there were no statistically significant changes in HbA1c values and health care utilization frequency, some clinical changes were documented. Specifically, changes in HbA1c values decreased, on average, by about a third of a percent, and there was a small overall drop in frequency of hospitalizations per participant in the 6 months after initiating treatment. Little and Rohlfing (2011) suggest that when evaluating impact of new treatments on HbA1c, a difference of at least 0.5% is needed to demonstrate a significant change. However, stability (i.e., a prevention of worsening) of HbA1c during the adolescent years is also clinically meaningful. Similarly, even a small decrease of one to two episodes in the frequency of hospitalizations for a subgroup of patients has large implications on the health care utilization costs when hospitalizations for diabetes ketoacidosis can cost up to $13,000 per single episode (Garg et al., 2004).
Therefore, this preliminary efficacy study showed that desired psychosocial outcomes were more likely to occur over time, but it is only known that these changes coincided with the intervention and not necessarily that the intervention influenced the change. Several other factors could account for this change as well, such as sampling bias, regression to the mean, and developmental maturation. A more extensive examination of the program among a larger sample is still warranted to demonstrate that these within-subject program effects were due to the intervention itself. It will also be important to document that the inability to find more effects for the between-groups analyses of the treatment versus the WLC groups were not due to program inefficacy, poor implementation, or low acceptance (Chambless & Ollen-dick, 2001; Flay, 1986).
It has been well established that HbA1c is one of the primary factors impacting long-term outcomes in diabetes (The DCCT Research Group, 1993). The observed decrease in HbA1c levels across time in this study was not statistically significant; however, the intervention in the present study showed a small to medium effect size for change over time for other diabetes-related factors (i.e., parental involvement in the division of diabetes responsibility and diabetes-related QOL), which have also been shown to be important to one's diabetes self-management (Kichler, Kaugars, Ellis, & Alemzahdeh, 2010). Increased parent involvement in the division of diabetes responsibility has been found to be a significant predictor of improved adherence to the diabetes regimen (Anderson et al., 2002), as parents take more of a “team” approach to sharing responsibility for the youth's T1DM management. Similarly, youths' diabetes-related QOL has been found to be significantly related to the presence of co-morbid depressive symptoms and poorer HbA1c levels (Lawrence et al., 2012).
Although this study did not demonstrate a statistically significant change in adolescents' HbA1c levels directly, the HbA1c levels did remain stable and even marginally decreased for many of the participants, on average, during the assessment year. Lack of improvement in glycemic control likely reflects the fact that multiple factors impact diabetes management (Danne et al., 2006). Stability in glycemic control, even when above the ideal range, over a 1-year period can keep hospitalization frequency and health costs down by preventing a worsening of HbA1c that is often seen throughout adolescence. Consistent with the existing literature, this study demonstrated an improvement on other modifiable individual and family diabetes-related factors over time using this treatment, which has important implications for making improvements in T1DM management and may potentially lead to better diabetes control and the prevention of long-term complications.
There were two statistically significant between-groups differences in the youth-reported general and diabetes-specific QOL for the treatment group at posttreatment and the WLC group immediately before the treatment was initiated (pretreatment). These scores suggested better QOL for the WLC control group participants than the treatment group at this time point. Given that there was no significant difference between the treatment and WLC control groups on general and diabetes-specific QOL at baseline assessment prior to randomization, this difference between the two groups of youth may be due to the attrition of four participants from the WLC group in the time frame between the baseline and pretreatment assessments. Therefore, future studies may want to make additional efforts to track participants who drop out of treatment to determine if there are any factors that led to their attrition. This also highlights the challenge of engaging families in psychosocial treatment when they may not be able to access services immediately.
There are several limitations to this study. This study was conducted with a clinical sample and was a pilot study with a small sample size. Only small and medium effect sizes were documented. The significant changes that were found over time cannot be directly attributed to the intervention, as other factors could also account for the changes found. Although there were no statistically significant differences between the treatment and WLC groups on demographic variables at baseline, there were differences in measures assessing psychosocial functioning and diabetes-related functioning. In order to control for these differences, those variables were included as covariates in the between-group MANCOVA analysis. Despite random assignment to groups and statistical control, these groups may have responded differently to the intervention over time. Therefore, the between-groups findings should also be interpreted cautiously. Given that some of the scores between the primary caregiver and adolescent were significantly correlated, independence of all of the variables cannot be assumed. Even though MANCOVA analyses do not require the assumption of sphericity, the results from the present study could be inflated due to the potential impact of parent and youth responses on one another. Therefore, the within-group findings should also be interpreted cautiously. In addition, there was a higher rate of attrition in the WLC group than the treatment group, which also impacts the generalizability of the findings to a subset of individuals who followed through with the intervention. The intervention is designed to be six sessions for all participants, and it may be that the treatment needs to be lengthened/shortened or a longer follow-up posttreatment time should be planned to demonstrate the impact of the intervention over time. Despite these limitations, this intervention appears to be a treatment modality that is feasible, acceptable, and adaptable in a clinical setting with adolescents who have T1DM and deserves further evaluation to determine efficacy and effectiveness over time. Notably, the setting for this group therapy intervention (i.e., within the context of routine outpatient mental health care) places this intervention in the unique position of being able to be replicated in other clinical settings by licensed psychologists and trainees.
Future clinical research needs to expand the evidence base for this treatment intervention by determining if there are age and gender differences by including an even wider age range of youth with T1DM and comparing across genders. Given the attrition in the WLC group and the positive effects evident at the 4-month post-treatment follow-up, it may not be necessary to utilize the WLC methodology, and future research studies may want to enroll all adolescents in the intervention together to be able to have enough statistical power to examine the within-group effects of the treatment over time. Finally, this intervention focused on adolescents who had been diagnosed with T1DM for at least 6 months. Even though the adolescents' length of diagnosis was not found to be related to outcomes, it may be that future studies may want to include patients with different lengths of time since diagnosis to determine if the intervention has a greater impact for certain sub-populations. Overall, future research should expand the knowledge base regarding this intervention by enrolling larger participant samples, utilizing wider age ranges of adolescents and preadolescents, recruiting adolescents newly diagnosed with T1DM, discontinuing the WLC design to minimize attrition, and following-up for a longer time period after the intervention. Attention to these factors will allow researchers to increase the evidence base for this treatment, thereby establishing that the intervention meets the criteria for probably efficacious and eventually well-established treatment in a clinical setting.
Footnotes
Body mass index standard deviation scores (BMI SDS) are calculated using the following equation: (BMI - 〈BMI〉)/SD, where BMI is weight/height2, and 〈BMI〉 and SD are the mean BMI and standard deviation for a specific age (Nigrin & Kohane, 1999).
Contributor Information
Jessica C. Kichler, Behavioral Medicine and Clinical Psychology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio and University of Cincinnati.
Astrida S. Kaugars, Department of Psychology, Marquette University.
Patricia Marik, Child and Adolescent Psychiatry and Behavioral Medicine, Children's Hospital of Wisconsin, Milwaukee, Wisconsin.
Laura Nabors, College of Education, Criminal Justice, and Human Services, University of Cincinnati.
Ramin Alemzadeh, Department of Pediatrics, University of Illinois at Chicago.
References
- Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LMB. Family conflict, adherence, and glycemic control in youth with short duration type 1 diabetes. Diabetic Medicine. 2002;19:635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Wolf MT, Burkhart RG, Cornell RG, Bacon GE. Effects of peer-group intervention on metabolic control of adolescents with IDDM. Randomized outpatient study. Diabetes Care. 1989;12:179–183. doi: 10.2337/diacare.12.3.179. [DOI] [PubMed] [Google Scholar]
- Boardway RH, Delamater AM, Tomakowsky J, Gutai JP. Stress management training for adolescents with diabetes. Journal of Pediatric Psychology. 1993;18:29–45. doi: 10.1093/jpepsy/18.1.29. [DOI] [PubMed] [Google Scholar]
- Boland EA, Grey M, Oesterle A, Frederickson L, Tamborlane W. Continuous subcutaneous insulin infusion: A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care. 1999;22:1779–1784. doi: 10.2337/diacare.22.11.1779. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Ollendick TH. Empirically supported psychological interventions: Controversies and evidence. Annual Review of Psychology. 2001;52:685–716. doi: 10.1146/annurev.psych.52.1.685. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Danne T, von Schutz W, Lange K, Nestoris C, Datz N, Kordonouri O. Current practice of insulin pump therapy in children and adolescents—The Hannover recipe. Pediatric Diabetes. 2006;7:25–31. doi: 10.1111/j.1399-543X.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Delamater AM, Jacobson AM, Anderson B, Cox D, Fisher L, Lustman P, Wysocki T. Psychosocial therapies in diabetes: Report of the psychosocial therapies working group. Diabetes Care. 2001;24:1286–1292. doi: 10.2337/diacare.24.7.1286. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. BSI: Brief Symptom Inventory. Administration, scoring and procedures manual. 4th. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with Type 1 diabetes in chronic poor metabolic control. A randomized control trial. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Flay BR. Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Preventive Medicine. 1986;15:451–474. doi: 10.1016/0091-7435(86)90024-1. [DOI] [PubMed] [Google Scholar]
- Garg SK, Walker AJ, Hoff HK, D'Souza AO, Gottleib PA, Chase HP. Glycemic parameters with multiple daily injections using insulin glargine versus the pump. Diabetes Technology & Therapeutics. 2004;6:9–15. doi: 10.1089/152091504322783350. [DOI] [PubMed] [Google Scholar]
- Greco P, Pendley JS, McDonell K, Reeves G. A peer group intervention for adolescents with Type 1 diabetes and their best friends. Journal of Pediatric Psychology. 2001;26:485–490. doi: 10.1093/jpepsy/26.8.485. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland E, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane W. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21:902–908. doi: 10.2337/diacare.21.6.902. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Chadwick MW, Schimmel LE. Social learning intervention to promote metabolic control in type I diabetes mellitus: Pilot experiment results. Diabetes Care. 1985;8:152–155. doi: 10.2337/diacare.8.2.152. [DOI] [PubMed] [Google Scholar]
- Kaugars A, Kichler J, Alemzadeh R. Assessing readiness to change the balance of responsibility for managing type 1 diabetes mellitus: Adolescent, mother, and father perspectives. Pediatric Diabetes. 2011;12:547–555. doi: 10.1111/j.1399-5448.2010.00737.x. [DOI] [PubMed] [Google Scholar]
- Kichler JC, Crowther JH. The effects of maternal modeling and negative familial communication on women's eating attitudes and body image. Behavior Therapy. 2001;32:443–457. doi: 10.1016/S0005-7894(01)80030-7. [DOI] [Google Scholar]
- Kichler JC, Kaugars AS, Ellis J, Alemzadeh R. Exploring self-management characteristics in youths with type 1 diabetes mellitus: Does membership in a glycemic control category matter? Pediatric Diabetes. 2010;11:536–543. doi: 10.1111/j.1399-5448.2010.00638.x. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Swales T, Klemp S, Madigan S. Proceedings of the Ninth Annual Sessions of the Society of Behavioral Medicine. Boston, MA: 1988. Self-care behaviors among adolescents with diabetes; p. A2. [Google Scholar]
- Lawrence JM, Yi-Frazier JP, Black MH, Anderson A, Hood K, Imperatore G SEARCH for Diabetes in Youth Study Group. Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. The Journal of Pediatrics. 2012;161:201–207. doi: 10.1016/j.jpeds.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RR, Rohlfing CL. Analytical goals for HbA1c: Are HbA1c results good enough for optimal use? Journal of Diabetes. 2011;3:3–6. doi: 10.1111/j.1753-0407.2010.00109.x. [DOI] [PubMed] [Google Scholar]
- Mendez FJ, Belendez M. Effects of a behavioral intervention on treatment adherence and stress management in adolescents with IDDM. Diabetes Care. 1997;20:1370–1375. doi: 10.2337/diacare.20.9.1370. [DOI] [PubMed] [Google Scholar]
- Moher D, Shulz K, Altman D. The CONSORT Statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. Annals of Internal Medicine. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- Nigrin DJ, Kohane IS. Society of Pediatric Research. San Francisco, CA: 1999. GrowthCalc: An anthropometric Calculator accessible via the World Wide Web. Retrieved from www.growthcalc.chip.org. [Google Scholar]
- Opipari-Arrigan L, Kichler J, Fredericks E, Burkhart N, Dale L, Eder S, Foster C. Published abstract at the American Diabetes Association 65th Scientific Sessions. San Diego, CA: 2005. Self-management intervention improved diabetes-related functioning in at-risk adolescents with type 1 diabetes. [Google Scholar]
- Plante WA, Labato D, Engle R. Review of group interventions for pediatric chronic conditions. Journal of Pediatric Psychology. 2001;26:435–453. doi: 10.1093/jpepsy/26.7.435. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children manual. 2nd. Minneapolis, MN: NCS Pearson, Inc.; 2000. [Google Scholar]
- Stark LJ, Mulvihil MM, Powers SW, Jelalian E, Keating K, Creveling S, Hovell MF. Behavioral intervention to improve calorie intake of children with cystic fibrosis: Treatment versus wait list control. Journal of Pediatric Gastroenterology and Nutrition. 1996;22:240–253. doi: 10.1097/00005176-199604000-00005. [DOI] [PubMed] [Google Scholar]
- The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitu. The New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in Type 1 and Type 2 diabetes: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Type 1 Diabetes Module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin P. The PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core scaled in healthy and patient populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: Preliminary reliability and validity. Health and Quality of Life Outcomes. 2004;2:55. doi: 10.1186/1477-7525-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, White N. Randomized trial of behavioral outcomes in adolescents: Maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30:555–560. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris M, Greco P, Bubb J, Danda CE, Harvey LM, White N. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25:23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]