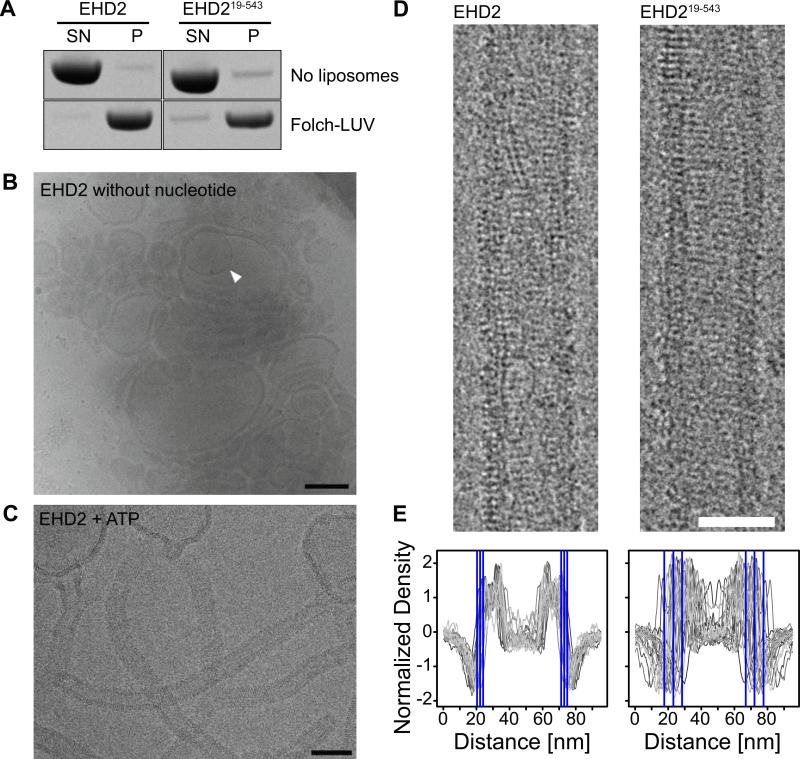
Figure 5. The N-terminus does not affect membrane-binding and oligomerization.
A) Co-sedimentation assays of EHD2 and EHD219-543 in the absence (upper panel) and presence (lower panel) of 800 nm filtered Folch-LUVs, without addition of nucleotides.
B, C) CryoEM of EHD2 in the presence of 800 nm Folch LUVs and absence of nucleotide (B) and in the presence of ATP (C). Inner vesicles of occasional multi-lamellar vesicles (B, white arrow) were shielded from the EHD2-containing buffer and showed typical bilayer structures, indicating that the surrounding liposomes were densely coated by EHD2. Similar to cryo electron micrographs of dynamin (Danino et al., 2004), we did not observe an accumulation of small vesicles, suggesting that EHD2 is not fragmenting liposomes under these conditions. Scale bar in B) 200 nm, in C) 100 nm.
D) CryoEM images of membrane tubules decorated with EHD2 and EHD219-543 were prepared by incubating EHD2 with Folch-LUVs in the presence of ATP. Regular patterns, most likely corresponding to ordered assemblies of the protein on the lipid tubule surface, were observed for both constructs. Scale bar is 50 nm.
E) 1D density profiles of membrane tubules decorated with EHD2 and EHD219-543. In case of EHD2, the average outer diameter of protein-coated tubules was similar (d=51±4 nm, n=2,156), as shown by the small standard deviation (SD). Deletion of the N-terminus had little effect on the average diameter of the tubules, but significantly increased the spread (d=49 ±11 nm, n=1,081). The blue lines in the 1D profile indicate the average outer limit of the tubes ± 1 SD.