Abstract
Aim
Rats selectively bred for inborn Low Capacity of Running (LCR) display a series of poor health indices where as rats selected for High Capacity of Running (HCR) display a healthy profile. We hypothesized that selection of low aerobic capacity over generations leads to a phenotype with increased diastolic Ca2+ leak that trigger arrhythmia.
Methods
We used rats selected for HCR (N=10) or LCR (N=10) to determine the effect of inborn aerobic capacity on Ca2+ leak and susceptibility of ventricular arrhythmia. We studied isolated FURA2/AM loaded cardiomyocytes to detect Ca2+-handling and function on an inverted epi-fluorescence microscope. To determine arrhythmogenicity we did a final experiment with electrical burst pacing in Langendorff perfused hearts.
Results
Ca2+-handling was impaired by reduced Ca2+ amplitude, prolonged time to 50% Ca2+ decay, and reduced sarcoplasmic reticulum (SR) Ca2+-content. Impaired Ca2+ removal was influenced by reduced SR Ca2+ ATP-ase 2a (SERCA2a) function and increased sodium/Ca2+-exchanger (NCX) in LCR rats. Diastolic Ca2 leak was 87% higher in LCR rats. The leak was reduced by CaMKII inhibition. Expression levels of phosphorylated theorine-286 CaMKII levels and increased RyR2 phosphorylation at the Serine-2814 site mechanistically support our findings of increased leak in LCR. LCR rats had significantly higher incidence of ventricular fibrillation.
Conclusion
Selection of inborn low aerobic capacity over generations leads to a phenotype with increased risk of ventricular fibrillation. Increased phosphorylation of CaMKII at serine-2814 at the cardiac ryanodine receptor appears as an important mechanism of impaired Ca2+ handling and diastolic Ca2+ leak that results in increased susceptibility to ventricular fibrillation.
Introduction
The strong statistical association between low aerobic capacity and increased risk for development of complex diseases is suggestive of a causal link. In 1996 Koch and Britton started testing for such linkage by selectively breeding to produce low and high strains of rats that contrast for inborn (i.e. untrained) aerobic endurance treadmill running capacity(Koch and Britton, 2001).
The hypothesis that rats selected for low capacity (Low Capacity Runners: LCR) would display negative health indices and the rats selected for high capacity (High Capacity Runners: HCR) would have positive health indices. After 11 generations of selection the LCR developed characteristics of the human condition know as metabolic syndrome relative to the HCR; the LCR rats have impaired cardiac function, elevated mean blood pressure, depressed endothelial function, insulin resistance, impaired glucose tolerance, increased visceral adiposity, higher plasma triglycerides, and elevated plasma free fatty acids(Wisloff et al., 2005).
Consistent with a poor health indices, the LCR also demonstrate diminished longevity compared to the HCR. Generation 20 of the LCR group died at a median age of 24.3 months and HCR died at 34.7 months, representing a 45% (10.7 months) difference in longevity (Koch et al., 2011). Increased RyR2 Ca2+ sensitivity that leads to increased frequency of spontaneously Ca2+ release from the SR during diastole seems to be a central feature of cardiac dysfunction and heart failure(Ai et al., 2005). This increase in spontaneous Ca2+ release may in turn trigger delayed after depolarization (DAD)(Pogwizd et al., 2001), ventricular arrhythmias, and sudden cardiac arrests(Anderson, 2004, Sag et al., 2009, van Oort et al., 2010).
Here we tested the hypothesis that contrasting for low intrinsic aerobic capacity over generations leads to a phenotype with increased Ca2+ leak over the ryanodince receptors that causes a more arrhythmogenic heart. Indeed, hearts from LCR demonstrated marked susceptibility to induction of ventricular fibrillation relative to hearts from HCR. Furthermore, in heart failure the activity of CaMKII is up regulated(Hoch et al., 1999, Kirchhefer et al., 1999), and several studies support that this may cause hyperphosphorylation of the ryanodine receptors 2 (RyR2) in the heart that causes spontaneously Ca2+-release. Our results support the notion that increased phosphorylation of RyR2 by CaMKII causes increased Ca2+-leak and susceptibility to ventricular arrhythmia in LCR rats.
Material and Methods
The animals used in the present study were senescent rats (two years of age) artificially selected and bred for high aerobic running capacity over 21 generations(HCR, N=10) and low aerobic running capacity(LCRs, N=10). The selection started out from a founder population of the genetically heterogeneous N:NIH stock of rats obtained from the National Institutes of Health (USA). The model system was previously described in detail(Koch and Britton, 2001). The Norwegian council for Animal Research approved the study, which was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996).
To determine the threshold for inducible arrhythmias we performed ex vivo experiments using a standardized Langendorff-perfusion. After obtaining stable recordings of ECG, we started electrical burst pacing of the left ventricle by a mini coaxial stimulation electrode with pacing lasting for 2 seconds with a 2 ms pulse width at a stimulation frequency of 20 Hz. The burst pacing was gradually increased from 0.2mA in 1mA steps every 20 seconds until VF threshold was reached, or to a maximal stimulation current of 32mA. Isolated Fura-2/AM-loaded cardiomyocytes were stimulated by bipolar electric pulses for Ca2+-handling and cardiomyocyte function by epi-fluorescence microscopy. Specific CaMKII and protein kinase A inhibitors were used. Confocal imaging of cardiomyocytes loaded with Fluo-3/AM were used to determine Ca2+-release synchrony and frequency of Ca2+ waves and transverse (T)-tubules were studied using the Di-8-ANEPPS(Stolen et al., 2009). Quantitative measurements of diastolic Ca2+-leak from the SR were performed on an inverted epi-fluorescence microscope by the method established by Shannon et al. (Shannon et al., 2002). To bring the cellular Ca2+-content to a steady state, we stimulated the cardiomyocytes electrically at 1 Hz in normal HEPES based 1.8 mmol/LCa2+ concentration solution for 30–60 seconds. After the last electric stimulus, we rapidly switched the perfusion to a 0Na+/0Ca2+ containing solution and measured diastolic Ca2+ concentration in quiescent non-stimulated cardiomyocytes and thereafter repeated the recordings in 0Na+/0Ca2+ solution with tetracaine (1 mmol/L). SR Ca2+ -content was measured by application of caffeine (10 mmol/L).
To assess SERCA-2a and NCX function we used the rate constant of Ca2+-decline in three different solutions to quantify the contribution from each factor; a) rate constant of Ca2+-decline during electrical stimulation in normal HEPES 1.8mmol/L Ca2+-solution, b) addition of 10 mmol/L Caffeine in normal HEPES 1.8 mmol/L Ca2+-solution, and c) addition of 10 mmol/L Caffeine in a 0Na+/0Ca2+ solution. The rate constant of Ca2+-decline in each situation was controlled for total SR Ca2+-content.
Mitochondrial respiration was studied in situ in saponin permeabilized fibers as described by Veksler and colleagues(Veksler et al., 1987). Maximal ADP-stimulated respiration was measured by addition of 2 mmol/L ADP as phosphate acceptor and the maximal respiration rate (Vmax) was calculated as (V0+VADP). Western blot analyses were performed as previously described (Stolen et al., 2009) for phosphorylated Threonine286-CaMKII, RyR2 (Affinity Bioreagents, Golden, CO), phosphorylated pRyR2 Serine2808 and phosphorylated pRyR2 serine2814 (These antibodies were a kind gift from Dr. Xander Wehrens, Houston, Texas, and previously described in (Respress et al., 2012). The use of Serine2808 and Serine2814 in the current paper refer to the human phosphorylation sites at the RyR2; Serine2808 equates to Serine2798 in rat RyR2 and Serine2814 in human RyR2 equates to Serine2804 in rat RyR2 (GenBank ID RyR2 RAT:NM_032078).
Independent samples t-test was used to determine the statistical differences between the groups. To test group differences in pacing induced ventricular fibrillation Fisher's exact test was used. P < 0.05 was considered statistically significant. Data are presented as mean ± SD unless otherwise stated. Detailed description of methods is presented in online supplements.
Results
Cardiomyocyte function and Ca2+ handling
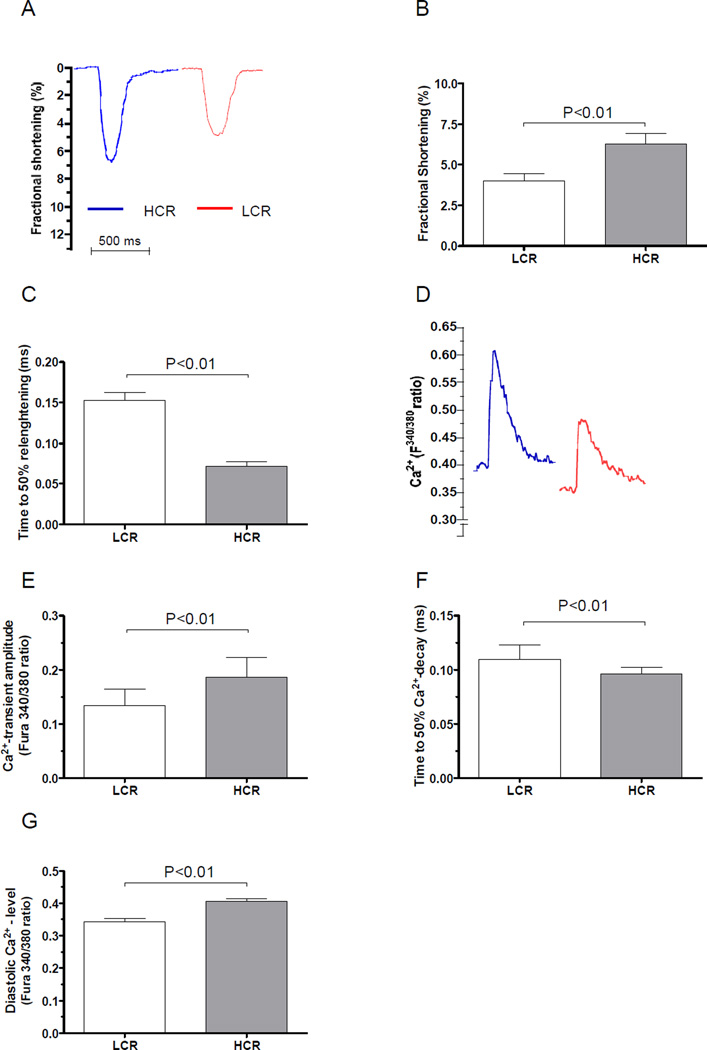
Cardiomyocyte contractility, measured as fractional shortening was reduced by 37% in LCR compared to HCR (Figure 1A-B, P < 0.01). Diastolic cardiomyocyte function measured as time to 50% relenghtening was prolonged in cardiomyocytes from LCR rats (Figure 1C, P < 0.01)
Figure 1. Cardiomyocyte function and Ca2+ handling properties.
(A) Representative sample tracings of cardiomyocyte fractional shortening from LCR (N=5) and HCR rats (N=5). (B) Fractional shortening was significantly reduced in LCR rats. (C) Time to diastolic relenghtening was longer in LCR rats. (D) Representative traces of Fura-2/AM ratio (F340/380). (E) Ca2+- transient amplitude (Fura-2/AM ratio F340/380) was lower in LCR rats. (F) Time to 50% Ca2+-decay was longer in LCR rats. (G) Diastolic Ca2+-level was lower in LCR rats.
We found a 28% lower Ca2+-transient amplitude(Figure 1D & E) in cardiomyocytes from LCR rats compared to HCR, which is in line with the reduced cardiomyocyte fractional shortening. Furthermore, the time to 50% Ca2+-decay during diastole was significantly longer in LCR rats, which accompany the defective diastolic function in these rats (Figure 1F, P < 0.01). Despite the longer time to 50% Ca2+-decay we were surprised to find lower end-diastolic Ca2+-concentration level during twitch contractions in LCR (Figure 1G, P < 0.01), which may be caused by a Ca2+-efflux from the cell over the sarcolemma by the NCX.
Diastolic SR Ca2+-leak
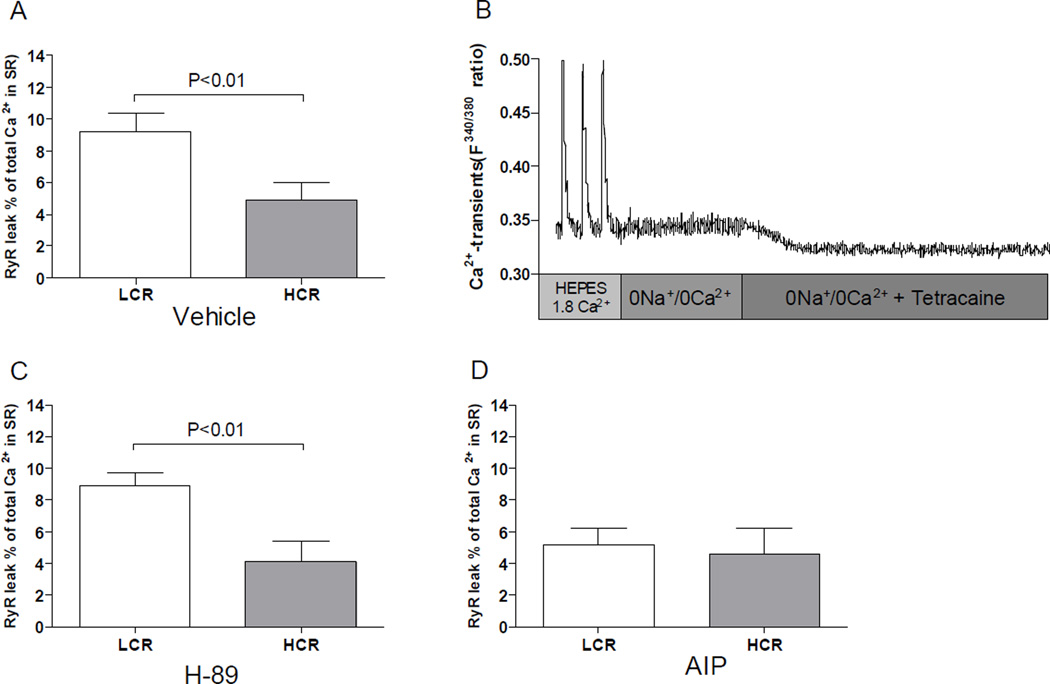
We detected 83% more SR Ca2+-leak during diastole in LCR rats compared to HCR (Figure 2A, P < 0.01 and Figure 2B). Increased SR Ca2+-leak during diastole can trigger delayed after depolarisations (DADs) that also were found more frequent in cardiomyocytes from LCR rats (29% of LCR cells vs. none in HCR, P < 0.01)(Figure 1 Online supplements). The increased SR Ca2+-leak probably reflects an increased RyR2 Ca2+-sensitivity which earlier studies link to increased phosphorylation at Serine-2808/9 by PKA or at Serine-2814 by CaMKII. To examine the effect of PKA and CaMKII we incubated separate cardiomyocytes with specific inhibitors. We did not find any effect of SR Ca2+-leak by the PKA inhibitorH-89, implying no involvement of this kinase (Figure 2C). When the cells were incubated with the specific CaMKII inhibitor AIP, the SR Ca2+- leak was almost abolished, implying that the RyR2 may be more sensitized by increased phosphorylation of CaMKII (Figure 2D).
Figure 2. Diastolic SR Ca2+ leak over the ryanodine receptor (RyR2).
(A) Diastolic SR Ca2+ leak in normal HEPES 1.8 Ca2+ concentration solution in LCR and HCR rats. (B) Exemplary recordings of diastolic SR Ca2+-leak in a LCR rat. (C) After incubation by AIP and (D), after inhibition by H-89. Note that LCR rats had significantly more diastolic SR Ca2+-leak than HCR rats in vehicle situation. In addition, inhibition of CamKII by AIP reduced the leak to of the LCR to equal that of HCR rats, but AIP had no further effect on HCR rats. PKA inhibition by H-89 did not have any significant effect in either of the groups. Number of rats used for cell experiments: LCR=5, HCR=5.
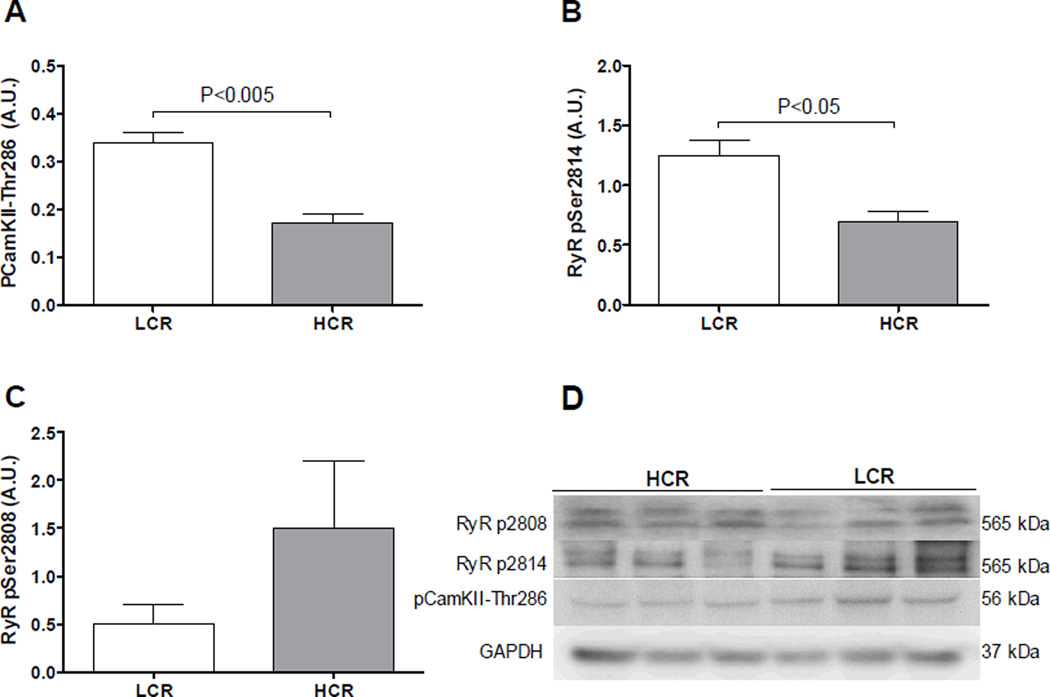
To follow up these findings, protein analyzes showed a 136% increased phosphorylation at the auto activating pCaMKII-threonine286site in the LCR rats (Figure 3A, P < 0.05) which imply increased activity of the kinase. Furthermore, the RyR2 was found significantly more phosphorylated at the CaMKII specific phosphorylation site serine2814 in the LCR (Figure 3B, P < 0.05). The PKA site serine2808 at the RyR2was not significantly different phosphorylated between LCR and HCR rats. (Figure 3C, NS). Taken together these data imply that phosphorylation of the RyR2 by CamKII is causing the increased diastolic SR Ca2+-leak in the LCR rats. This increased SR Ca2+-leak may contribute to the 20% reduced SR Ca2+-content found in LCR rats (Figure 4A, P < 0.01)
Figure 3. Protein expression from left ventricle tissue.
Western blots from left ventricle tissue LCR and HCR rats. (A) The levels of phosphorylated CaMKII at the theorine-286 site were significantly higher in LCR rats than HCR. (B) Phosphorylation of the RyR2 at the serine-2814 site was increased in LCR rats. (C) Phosphorylation of the RyR2 at the serine-2808 site was lower, on average, in LCR but not significantly different from HCR. Data are presented as mean ± SEM.
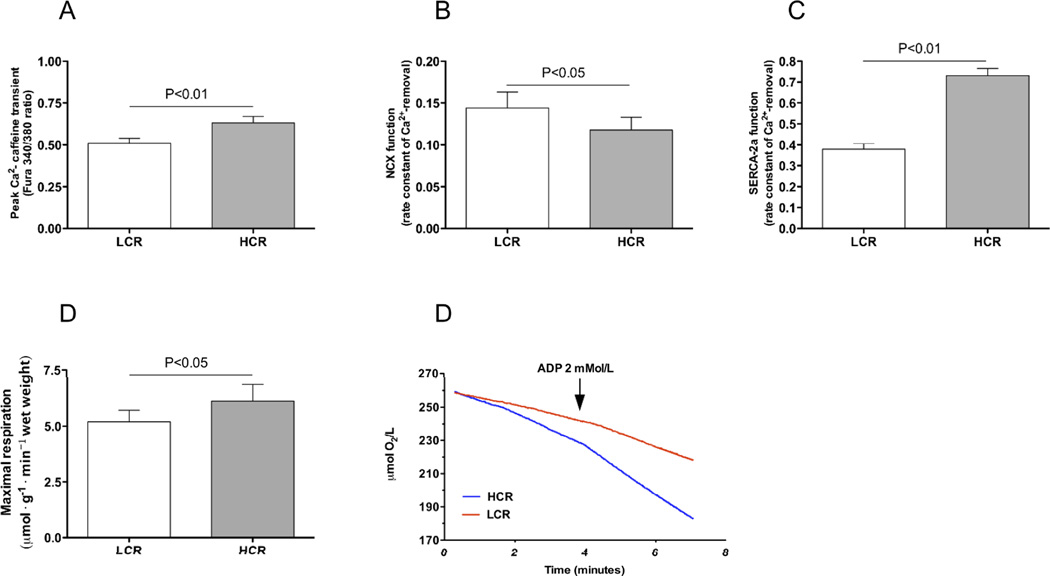
Figure 4. Sarcoplasmic reticulum, sarcolemmal Ca2+ handling and mitochondrial function.
(A) Total SR Ca2+-content was significant lower in LCR rats compared to HCR. (B) NCX function was increased in LCR rats assessed by the rate constant of Ca2+ removal(Fura2/AM ratio340/380· second−1),(C) SERCA-2a function was reduced in LCR rats. (D) Mitochondrial respiration by ADP-stimulation of respiration to maximal respiration in freshly dissected saponin permeabilized left ventricle tissue was significantly lower in LCR rats compared to HCR (determined by µmol O2 · g−1 · min−1 consumption). (E) Exemplary recordings from LCR (red) and HCR (blue). Number of rats used for experiments: LCR=5, HCR=5.
SERCA2a-, NCX-and mitochondrial function
To more carefully determine the Ca2+-fluxes during diastole we examined the rate constants of cytoplasmic Ca2+ removal from twitch-induced and caffeine-induced Ca2+ transients. We found that the Ca2+ removal rate of NCX was significantly higher in LCR rats (Figure 4B, P < 0.05) whereas SERCA2a Ca2+ removal rate was increased in LCR compared to HCR (Figure 4C, P < 0.01).
As SERCA-2a function is dependent upon the local ATP/ADP ratio in the pump’s vicinity (Kuum et al., 2009), we analyzed mitochondrial respiration in separate samples of tissue from the left ventricle to check for a potential effect of metabolism on Ca2+ removal. We found that LCR rats had a 23% lower maximal ADP stimulated respiration than HCR rats (Figure 4D & E, P < 0.05).
T-tubule density and Ca2+-release synchrony
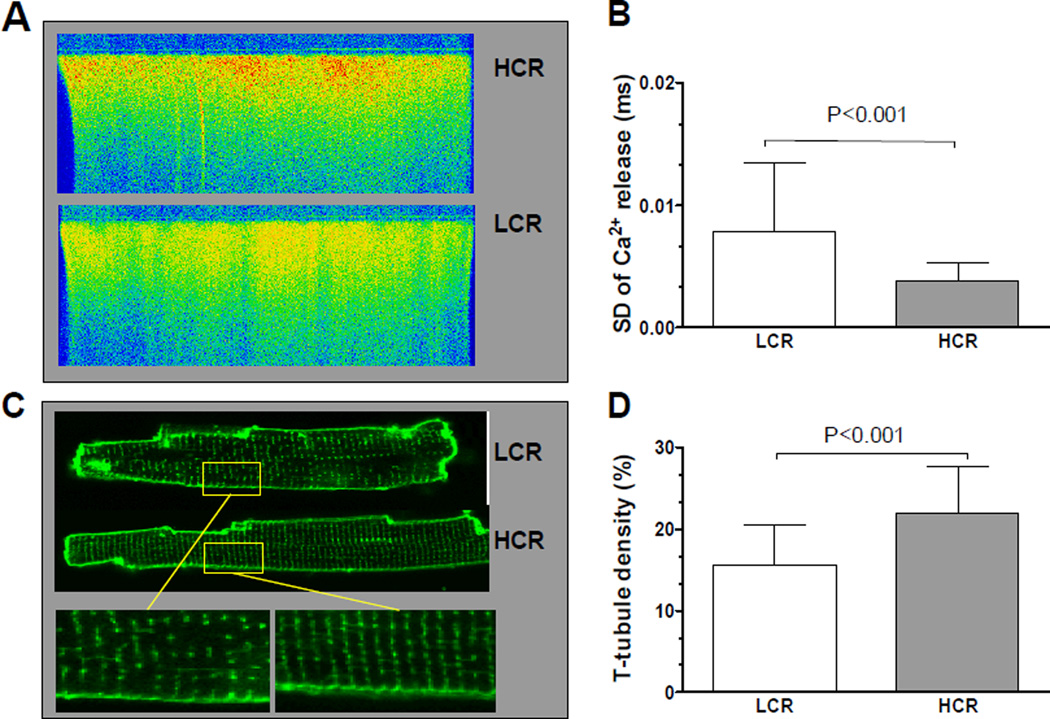
For detailed examination of Ca2+-release properties and synchrony of Ca2+ release along the cardiomyocyte length we performed line-scan confocal imaging in Fluo-3 loaded electrically stimulated cells. We found that cardiomyocytes from LCR rats had a less synchronous Ca2+ release compared to HCR cardiomyocytes (Figure 5A and B, P < 0.001). Synchrony of Ca2+ release is closely linked to the density and organization of T-tubules in the cardiomyocyte (Brette and Orchard, 2003, Lipp et al., 1996, Louch et al., 2004). In line with this, we found both reduced T-tubule density and distorted structure in LCR cardiomyocytes (Figure 5C and D). Additionally, we measured the distance between T-tubules to control for the possibility that the spacing between the T-tubules were altered. We found that the spacing between T-tubules(for intact T-tubules) tended to be longer in LCR, but did not reach statistical significance (1.84±0.18µm vs. 1.76±0.12µm, in LCR and HCR respectively, P=0.055). This indicates that the difference in T-tubule density was due to loss of T-tubules in small regions of the cell and not spacing between intact T-tubules.
Figure 5. Ca2+ release synchrony and T-tubule density.
(A) Exemplary recordings of Ca2+-release synchrony in Fluo-3 loaded electrically stimulated cardiomyocytes. (B) Quantification of Ca2+-release synchrony measured as the standard deviation (SD) of Ca2+-release along the line-scanned whole length of the cardiomyocyte showed significant more variation in the syncrony in LCR rats. (C) Exemplary images of Di-8-ANEPPS stained cardiomyocytes as measurement for T-tubule density in LCR and HCR rats. (D) Quantified measures of T-tubule density in di-8-ANEPPS stained cardiomyocytes revealed significantly reduced density in LCR rats. Number of rats used for cell experiments: LCR=5, HCR=5.
Propensity for burst pacing induced ventricular fibrillation
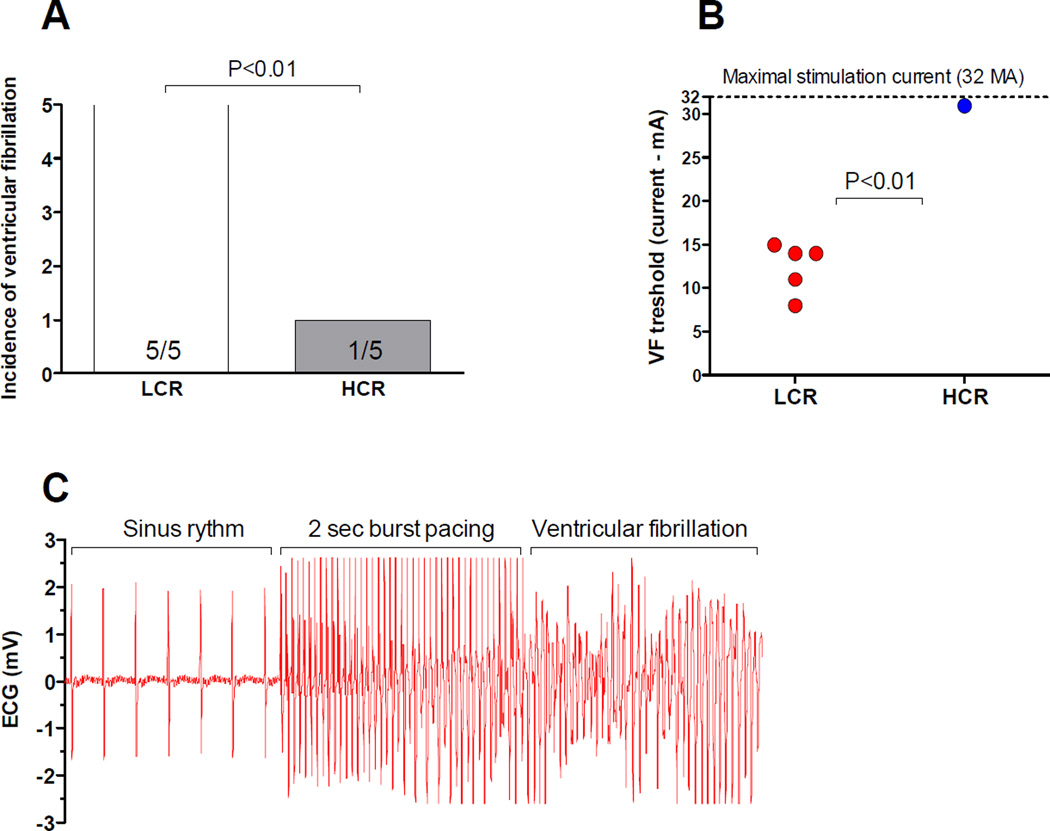
Finally, to asses if altered Ca2+ handling properties and especially the increased RyR2 Ca2+ leak observed had any effect upon the arrhythmogenicity of the heart we performed experiments by burst pacing of the left ventricle in Langendorff perfused hearts in sinus rhythm. We found that LCR rats were significantly more susceptible to ventricular fibrillation than HCR, with 5/5 hearts that experienced sustained ventricular fibrillations in LCR (threshold 12.4±2.9mA) compared to only 1/5 in HCR (Figure 6A-C, P < 0.01).
Figure 6. Aerobic capacity and incidence of ventricular fibrillation.
Recordings of ventricular fibrillation (VF) after burst pacing of the left ventricle. (A) LCR (N=5) rats were significantly more prone for sustained ventricular fibrillation than HCR rats (N=5). (B) LCR rats had a mean threshold for ventricular fibrillation of 12mA and in all measured hearts we managed to induce sustained fibrillation. Only one HCR heart sustained fibrillation at a current of 31mA. (C) Exemplary recordings of burst pacing induced ventricular fibrillation in a LCR heart.
Discussion
The present study was initiated to test the hypothesis that long-term artificial selection for contrasting intrinsic aerobic capacities would lead to senescent phenotypes that diverge for diastolic Ca2+ control that may cause a more arrhythmogenic heart. The results demonstrate that a low aerobic capacity harbors numerous negative cardiac phenotypes including: 1) depressed cardiomyocyte systolic and diastolic function, 2) increased diastolic SR Ca2+-leak over the RyR2associated with auto-phospohorylated CaMKII and increased RyR2 phosphorylation at the Serine-2814 site, and 3) increased propensity to ventricular arrhythmia.
Cardiomyocyte function and Ca2+ handling properties
Impaired cardiomyocyte function in LCR rats was associated with deterioration of Ca2+ handling properties including: 1) reduced systolic Ca2+-amplitude that could account for the reduced cardiomyocyte fractional shortening, and 2) the reduced removal of Ca2+ from cytosol during diastole that resulted in prolonged time to diastolic relenghtening of the cardiomyocyte. Several factors may causatively explain this prolongation, but the main route of Ca2+ removal is the SERCA-2a (Bers, 2002), which was found significantly impaired in the LCR rats. The combination of reduced SERCA-2a function and increased NCX function in LCR rats may further contribute to reduce SR Ca2+ content that was observed in LCR rats, since this earlier have been reported to cause higher Ca2+ extrusion over the cell membrane (Hasenfuss et al., 1999, Litwin and Bridge, 1997, Pogwizd et al., 2001, Sipido et al., 2000, Bers et al., 2006). Furthermore, loss of the T-tubular network causes disruption of the dyads and impairs EC-coupling by dys-synchronous Ca2+ release as observed in the LCR. The lower T-tubule density in the LCR was mainly caused by loss of T-tubules in small areas within the cardiomyocyte as the T-tubule spacing did not differ. Reduced T-tubule density/organization and in-efficient coupling is commonly observed in heart failure models, and has been associated with loss of the structural proteins junctophilin-2 and bin-1 (Hong et al., 2010, Lee et al., 2002, van Oort et al., 2011). However, how these essential proteins are regulated is unknown.
Increased diastolic SR Ca2+ leak and ventricular fibrillation in LCR rats
The cardiomyocyte functional data and Ca2+-handling measurements clearly pointed to a malfunctioning cardiomyocyte with disordered Ca2+ homeostasis. Because RyR2 instability has been associated with spontaneous Ca2+ release during diastole that may trigger DADs, depolarization of the cardiomyocyte, and subsequent ventricular arrhythmias (Pogwizd et al., 2001), we performed diastolic Ca2+-leak recordings in isolated cardiomyocytes.
In the presence of tetracaine, which de-sensitizes the RyR2, we were able to detect a significant increased Ca2+-leak in LCR rat compared to HCR rats. It has earlier been shown that increased RyR2 Ca2+-leak is associated with triggering of DADs through activating forward mode of the NCX, leading to a net ion influx. Based upon studies from heart failure models it have been shown that increased activity of CaMKII may lead to increased phosphorylation of the RyR2 and hence increased Ca2+-sensitivity of the receptor making it more “leaky”. To determine the role of CaMK as a potential mechanistic explanation to the increased SR Ca2+ leak observed in LCR rats, we used the specific CaMKII inhibitor AIP. The results displayed a significant reduction of Ca2+ leak by AIP inhibition of CaMKII. These data indicate an important role of CaMKII as a determinant of the increased leak in LCR rats in the present study. In 2000 Marks et al. proposed a new theory that PKA hyperphosphorylates the RyR2 at serine2808 site causing FK506 binding protein 12.6 (FKBP 12.6) dissociation from the receptor and diastolic SR Ca2+ leak. These initial data was followed up by series of highly consistent and compelling data published in prominent journals that supported their hypothesis (Wehrens et al., 2003, Wehrens et al., 2006, Wehrens et al., 2004). The hypothesis have however been challenged in later years since several independent laboratories have been unable to reproduce the findings, rising a series of controversies and debate of its fundamental role in causing SR Ca2+ leakage (reviewed in eg. (Bers, 2012, Eschenhagen, 2010, Valdivia, 2012)). In light of the controversies on the potential role of PKA dependent influence on the RyR2 SR Ca2+ leak and the fact that various experimental animal models may display different mechanisms, we opened the possibility as an explanation for the increased SR Ca2+ leak observed in LCR rats. To determine the effect of PKA on reducing the higher SR Ca2+ leak in LCR rats we therefore performed a set of experiments using the PKA inhibitor H-89. The PKA inhibition had no significant effect on the diastolic SR Ca2+ leak compared to vehicle solution. The data from our model of LCR rats is therefore in line with previous data from heart failure models that question the role of PKA for influencing RyR2 Ca2+-leak and rather designate CaMKII as a key effector of the increased RyR2 Ca2+-leak (reviewed in eg. (Bers, 2012, Eschenhagen, 2010, Valdivia, 2012).
Given the marked effect on reduction of Ca2+-leak by the inhibition of CaMKII we hypothesized that the LCR rats had increased activity of CaMKII that indeed was confirmed by increased expression of threonine286 phosphorylation of CaMKII (pCaMKII-Threonine286) in LCR rats. pCaMKII-Threonine286 increases the affinity of the CaMKII complex and traps CaMKII on the auto phosphorylated subunit. As a result, the kinase retains close to fully activity as long as CaM is trapped, regardless of the Ca2+-concentration level(Meyer et al., 1992). The effect of increased activity of CaMKII was also found in the increased phosphorylation status at the CaMKII specific site serine-2814 at the RyR2 in LCR. Hence, these data further substantiate that increased Ca2+-sensitivity of the RyR2is attributed to augmented CaMKII activity as a key player underlying the increased diastolic SR Ca2+-leak in the LCR rats.
The increased CaMKII activity caused by pCaMKII-threonine286 autophosphorylation is commonly observed in conditions of heart failure(Maier and Bers, 2007). We do not have any direct explanation to the increased CaMKII activation in the LCR rats in the present study, but several previous studies have shown that the LCR rat model display a series of characteristics indicative of numerous metabolic disease states including obesity, hyperlipidemia, impaired glucose tolerance and insulin resistance (Bowman et al., 2010, DeMarco et al., 2012, Haram et al., 2009, Lessard et al., 2009, Wisloff et al., 2005). Recently it was shown in an elegant study by Bers group that hyperglycaemia activates CaMKII by covalent modification of CaMKII by O-linked Nacetylglucosamine (O-GlcNAc). O-GlcNAc modification of CaMKII at Serine-279 activated further CaMKII autonomously trough increased phosphorylation of Threonine-286 (Erickson et al., 2013). To relate these findings to the increased CaMKII activity in the present study on LCR rats, the recent findings showing that LCR rats have augmented cardiac O-GlcNAc protein modification(Johnsen et al., 2013) are interesting and highly relevant and suggestive for a possible mechanistic explanation. Further studies are, however, needed to establish this link directly. In addition, the contrasting phenotypic differences observed between the LCR and HCR lines have in a previous publication been linked to a range of differently expressed genes in the heart (1540 out of 28000 screened genes), that influence signaling processes that potentially activates CaMKII, including eg. Ca2+ signaling and glucose metabolism pathways (Bye et al., 2008). The current experiment can, however, not in detail quantify how much of the breakdown of Ca2+ handling properties in the LCR that are directly affected by either the genetic differences or remotely as influenced by enhanced disease processes caused by a multitude of metabolic and pathologic processes of the senescence phase. A recent study showed, however, that the proportion of total running performance that is due to the additive effects of genes (i.e., narrow-sense heritability) is about 40% in each line (Ren et al., 2013). In addition, earlier studies have shown that the differences between HCR and LCR lines are significantly changed also in younger rats(Hoydal et al., 2007, Wisloff et al., 2005). This is consistent with the possibility that LCR-HCR phenotypic differences may be mediated via expression of contrasting allelic variants. Whether the cardiac differences are mediated directly via genetic variation or by remote disease influences, the models can still serve to evaluate interventions that retrieve or enhance negative phenotypes in the LCR. In this regard, earlier studies have shown that exercise training in LCR rats are to improve cardiomyocyte function as well as Ca2+ handling properties in LCR rats (Hoydal et al., 2007, Wisloff et al., 2005). It is therefore possible that preventive strategies, including exercise training, starting from young age and throughout life could induce a more advantageous health profile, prevent deterioration of cardiomyote properties and thereby improve longevity. This needs to be addressed in further studies.
Ventricular arrhythmias in non-ischemic hearts are mainly initiated by non re-entrant mechanisms that appears to be due to triggered activity primarily from DADs that arise from altered cellular Ca2+ handling and ionic currents (Pogwizd and Bers, 2004). As the measurements where recorded in isolated Langendorff perfused hearts, intrinsic properties of the ventricle determined the VF threshold. Recent literature has attributed an altered VF threshold to imbalanced Ca2+-homeostasis, increased diastolic SR Ca2+-leak in combination with altered electrogenic balance via increased NCX activity and elevated resting membrane potential (Pogwizd and Bers, 2004). It is therefore, highly possible that the increased SR Ca2+ leak, combined with the increased NCX activity are important players in the increased incidence of VF observed in the LCR rats.
In conclusion, during two-way selection for low and high aerobic running capacity increased susceptibility for disease risks segregated with low capacity. Here we show that the low aerobic capacity line also presents with increased propensity for ventricular fibrillation that was under written by impaired Ca2+ handling, increased diastolic SR Ca2+ leak over the RyR2 trough, and increased CaMKII activation and RyR2 phosphorylation at serine-2814.
Supplementary Material
Acknowledgements
We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan. Contact L.G.K. (lgkoch@med.umich.edu) or S.L.B. (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international collaborative resource at the University of Michigan, Ann Arbor. The authors acknowledge Ragnhild Røsbjørgen for technical assistance and isolation of cardiomyocytes.
Funding
This work was supported by the Department of Circulation and Medical Imaging at the Norwegian University of Science and Technology, The K. G. Jebsen Foundation and the Norwegian Council of Cardiovascular Disease. The LCR-HCR rat model system was funded by the National Center for Research Resources grant R24 RR017718 and is currently supported by the Office of Research Infrastructure Programs/OD grant ROD012098A (to L.G.K. and S.L.B.) from the National Institutes of Health. S.L.B. was also supported by National Institutes of Health grant RO1 DK077200.
Non-standard Abbreviations and Acronyms
- LCR
Low Capacity Runners
- HCR
High Capacity Runners
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DAD
delayed afterdepolarization
- NCX
Na+/Ca2+-exchanger
- PKA
protein kinase A
- PLB
phospholamban
- PMCA
plasma membrane Ca2+ ATPase
- RyR2
ryanodine receptor type 2
- SERCA2a
Sarcoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- T-tubules
transverse tubules
- VO2max
maximal oxygen uptake
Footnotes
Conflict of interest: none declared.
References
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–161. doi: 10.1016/j.tcm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and na+ in normal and failing cardiac myocytes. Ann N Y Acad Sci. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- Bowman TA, Ramakrishnan SK, Kaw M, Lee SJ, Patel PR, Golla VK, Bourey RE, Haram PM, Koch LG, Britton SL, Wisloff U, Lee AD, Najjar SM. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology. 2010;151:5157–5164. doi: 10.1210/en.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics. 2008;33:100–109. doi: 10.1152/physiolgenomics.00269.2007. [DOI] [PubMed] [Google Scholar]
- DeMarco VG, Johnson MS, Ma L, Pulakat L, Mugerfeld I, Hayden MR, Garro M, Knight W, Britton SL, Koch LG, Sowers JR. Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2012;302:H1667–H1682. doi: 10.1152/ajpheart.01027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T. Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure? J Clin Invest. 2010;120:4197–4203. doi: 10.1172/JCI45251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram PM, Kemi OJ, Lee SJ, Bendheim MO, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisloff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81:723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoydal MA, Wisloff U, Kemi OJ, Britton SL, Koch LG, Smith GL, Ellingsen O. Nitric oxide synthase type-1 modulates cardiomyocyte contractility and calcium handling: association with low intrinsic aerobic capacity. Eur J Cardiovasc Prev Rehabil. 2007;14:319–325. doi: 10.1097/hjr.0b013e3280128bef. [DOI] [PubMed] [Google Scholar]
- Johnsen VL, Belke DD, Hughey CC, Hittel DS, Hepple RT, Koch LG, Britton SL, Shearer J. Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity. Physiol Genomics. 2013;45:17–25. doi: 10.1152/physiolgenomics.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuum M, Kaasik A, Joubert F, Ventura-Clapier R, Veksler V. Energetic state is a strong regulator of sarcoplasmic reticulum Ca2+ loss in cardiac muscle: different efficiencies of different energy sources. Cardiovascular Research. 2009;83:89–96. doi: 10.1093/cvr/cvp125. [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150:4883–4891. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Huser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol. 1996;497(Pt 3):589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin SE, Bridge JH. Enhanced Na(+)-Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ Res. 1997;81:1083–1093. doi: 10.1161/01.res.81.6.1083. [DOI] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Ren YY, Overmyer KA, Qi NR, Treutelaar MK, Heckenkamp L, Kalahar M, Koch LG, Britton SL, Burant CF, Li JZ. Genetic analysis of a rat model of aerobic capacity and metabolic fitness. PLoS One. 2013;8:e77588. doi: 10.1371/journal.pone.0077588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Ginsburg KS, Bers DM. Quantitative Assessment of the SR Ca2+ Leak-Load Relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ, Vos MA. Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102:2137–2144. doi: 10.1161/01.cir.102.17.2137. [DOI] [PubMed] [Google Scholar]
- Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisloff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- Valdivia HH. Ryanodine receptor phosphorylation and heart failure: phasing out S2808 and "criminalizing" S2814. Circ Res. 2012;110:1398–1402. doi: 10.1161/CIRCRESAHA.112.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.