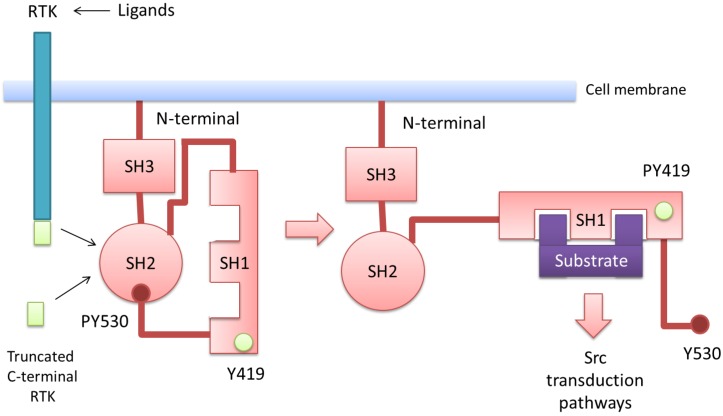
Figure 3.
Schematic representation of Src in the low activity state (left) and the active state (right). In the low activity configuration, the SH2 domain binds the phosphorylated C-terminal Tyr530, while the SH3 domain interacts with the linker domain, promoting a relative “closed” conformation. In the active configuration, SH2 and SH3 domains are released from the intramolecular interactions with autophosphorylation of Tyr419, which enhances the catalytic activity of Src. Activation of Src is mediated by activation of transmembrane tyrosine receptors (RTK) upon binding the corresponding ligands or, alternatively, by the C-terminal intracellular truncated isoforms.