Abstract
Objectives To review, summarise, and compare the evidence for effectiveness of screening sigmoidoscopy and screening colonoscopy in the prevention of colorectal cancer occurrence and deaths.
Design Systematic review and meta-analysis of randomised controlled trials and observational studies.
Data sources PubMed, Embase, and Web of Science. Two investigators independently extracted characteristics and results of identified studies and performed standardised quality ratings.
Eligibility criteria Randomised controlled trials and observational studies in English on the impact of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality in the general population at average risk.
Results For screening sigmoidoscopy, four randomised controlled trials and 10 observational studies were identified that consistently found a major reduction in distal but not proximal colorectal cancer incidence and mortality. Summary estimates of reduction in distal colorectal cancer incidence and mortality were 31% (95% confidence intervals 26% to 37%) and 46% (33% to 57%) in intention to screen analysis, 42% (29% to 53%) and 61% (27% to 79%) in per protocol analysis of randomised controlled trials, and 64% (50% to 74%) and 66% (38% to 81%) in observational studies. For screening colonoscopy, evidence was restricted to six observational studies, the results of which suggest tentatively an even stronger reduction in distal colorectal cancer incidence and mortality, along with a significant reduction in mortality from cancer of the proximal colon. Indirect comparisons of results of observational studies on screening sigmoidoscopy and colonoscopy suggest a 40% to 60% lower risk of incident colorectal cancer and death from colorectal cancer after screening colonoscopy even though this incremental risk reduction was statistically significant for deaths from cancer of the proximal colon only.
Conclusions Compelling and consistent evidence from randomised controlled trials and observational studies suggests that screening sigmoidoscopy and screening colonoscopy prevent most deaths from distal colorectal cancer. Observational studies suggest that colonoscopy compared with flexible sigmoidoscopy decreases mortality from cancer of the proximal colon. This added value should be examined in further research and weighed against the higher costs, discomfort, complication rates, capacities needed, and possible differences in compliance.
Introduction
Since 1992, several observational studies have suggested a major protective effect of lower gastrointestinal endoscopy (in particular sigmoidoscopy and colonoscopy) against colorectal cancer through detection and removal of precancerous lesions.1 2 3 As a result, and supported by further improvements in technology, use of sigmoidoscopy and colonoscopy for diagnostic and screening purposes has substantially increased in many countries.4 5 Both procedures have been recommended as screening options for colorectal cancer by expert committees and offered as primary screening in several European countries (including Germany, Italy, Poland, and Austria) long before the availability of results from randomised controlled trials.6 7 8 9 Recent studies of trends in incidence and mortality from colorectal cancer suggest that increased use of lower gastrointestinal endoscopy has already led to major reductions in the incidence of and deaths from colorectal cancer in the United States.10 11
Emerging evidence on the protective effects of endoscopy from detection and removal of adenomas also prompted the initiation of several randomised controlled trials to prove the efficacy of endoscopy based screening for reducing colorectal cancer incidence and mortality. In the 1990s four large scale randomised controlled trials began to investigate the effects of screening by flexible sigmoidoscopy, and the first results were published in 2009,12 2010,13 2011,14 and 2012.15 Screening colonoscopy has the potential to prevent colorectal cancer of the entire large bowel but is associated with higher costs, discomfort, complication rates, and capacities needed. Given that colonoscopy is recommended and offered for primary screening in an increasing number of countries, it is important to know its relative effectiveness compared with sigmoidoscopy. The only randomised controlled trial to assess the impact of screening colonoscopy (compared with no screening) on colorectal cancer incidence and mortality started recruitment in 2009, and the first results on reduction of colorectal cancer incidence and mortality are not expected before the mid-2020s.16
We reviewed, summarised, and compared the evidence from published randomised controlled trials and observational studies investigating the effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality in the population at average risk for colorectal cancer available to date.
Methods
We carried out a systematic literature review and meta-analysis according to a predefined protocol. Reporting follows the PRISMA and MOOSE statements.17 18
Data sources and searches
We searched PubMed, Web of Science, and Embase for eligible studies from inception to 7 November 2013 (for Embase until 2 August 2012 only, owing to termination of our institutional licence; until then, no additional relevant article had been retrieved through Embase). The combinations of keywords used were (“sigmoidoscopy” or “colonoscopy” or “endoscopy” or “polypectomy”) and (“colorectal” or “colon” or “large bowel”) and (“cancer”) and (“relative risk” or “relative risks” or “ratio” or “ratios” or “rate” or “rates”) and (“cohort” or “case control” or “trial” or “intervention” or “randomized” or “follow up”). We searched the reference lists of identified sources for additional relevant studies.
Study selection
Published randomised controlled trials and observational studies were eligible for inclusion if they assessed the effects of screening sigmoidoscopy or screening colonoscopy versus no endoscopy on colorectal cancer incidence or mortality, or both in the general population at average risk for colorectal cancer. The review was restricted to original articles published in English. We excluded studies published as abstracts only as we considered the information to be unsufficient for our assessment. Two reviewers (CS, MH) independently performed study selection according to eligibility criteria. Disagreements were resolved by discussion or a third reviewer (HB).
Data extraction and quality assessment
Two reviewers (HB, MH) independently extracted relevant information from both types of studies into a standardised form. Information extracted from randomised controlled trials was first author, year of publication, country, number and age range of participants, type of intervention, years of enrolment, and median follow-up time. In addition we extracted participation rates in the intervention group and contamination proportions (that is, the proportions undergoing lower gastrointestinal endoscopy in the control group) where reported. Information extracted from observational studies was first author, year of publication, country, years of colorectal cancer diagnosis or death, type of lower gastrointestinal endoscopy (sigmoidoscopy, colonoscopy), time frame of endoscopies, and covariates considered.
For both randomised controlled trials and observational studies we extracted estimates of relative risk along with 95% confidence intervals according to site of colorectal cancer (any, proximal, distal) and outcome (incidence, mortality) as far as reported. For randomised controlled trials we extracted the results from both the intention to screen and the per protocol analyses.
The two reviewers resolved disagreements in data extracted by further review and discussion.
A quality assessment of included studies was conducted for descriptive purposes and to evaluate potential differences in results according to quality criteria (see supplementary table 1 for details of the quality indicators). Two reviewers (HB, MH) independently assessed study quality and resolved disagreements by further review and discussion.
Data synthesis and analysis
We combined the studies in a narrative synthesis, focusing on differences in effect estimates according to study design, type of endoscopy, and cancer site. To simplify terminology, we uniformly refer to the effect estimates of relative incidence or mortality from randomised controlled trials and cohort studies and of odds ratios from case-control studies as estimates of “relative risk.” We calculated pooled effect sizes together with 95% confidence intervals using random effects models,19 stratified by study design (randomised controlled trials or observational) and type of endoscopy (screening sigmoidoscopy or screening colonoscopy). From reported confidence intervals we calculated standard errors using the delta method. Subgroup analyses were conducted according to cancer site and type of analysis (intention to screen, per protocol; randomised controlled trials only). We assessed heterogeneity in effect estimates using Ι², τ², and Cochran’s Q statistic.20 Publication bias was assessed by funnel plots.21 The Bucher method was applied to indirectly compare the effectiveness of sigmoidoscopy and colonoscopy for colorectal cancer screening.22
We used the “meta” package in R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) to perform meta-analyses.23
Results
Study selection
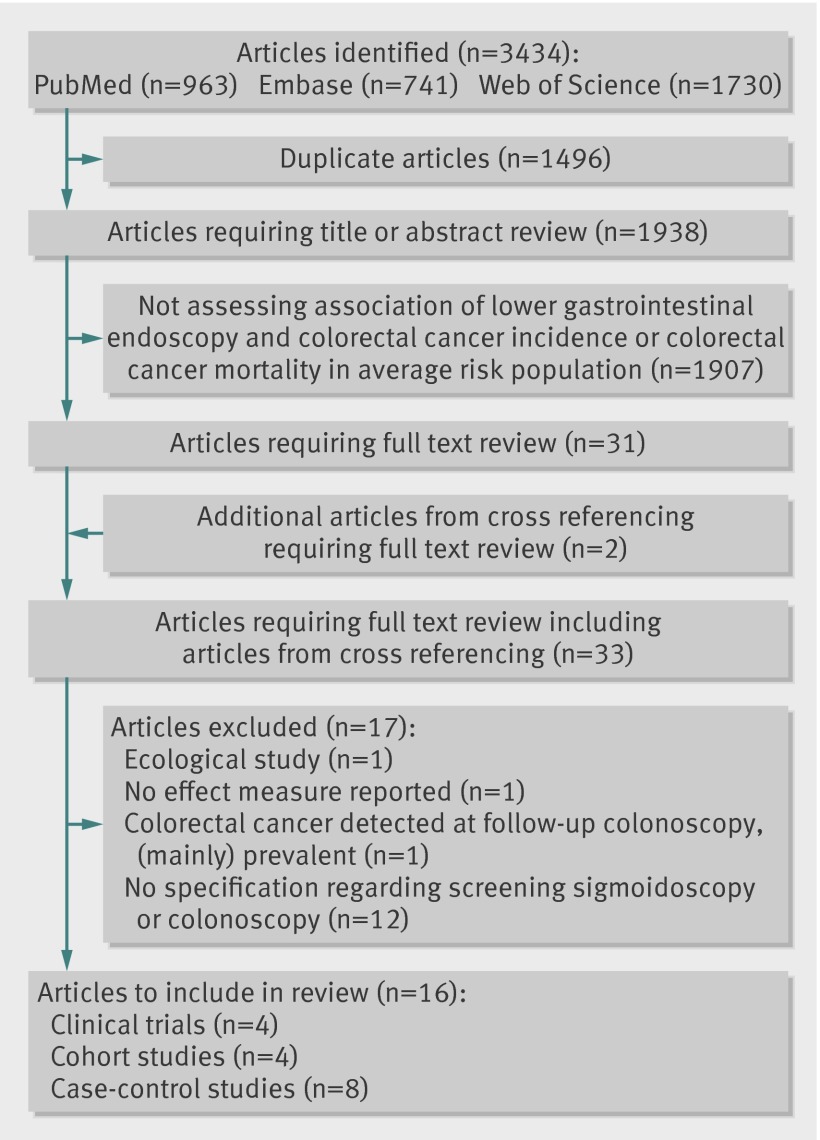
After full text review of 33 articles, we excluded one study assessing the association between colonoscopy use and colorectal cancer in an ecological approach—that is, on an aggregate (regional) level only (figure).24 Furthermore, we excluded two studies that reported no effect measures or only separate estimates of risk reduction after positive or negative sigmoidoscopy results.25 26 We also excluded one small randomised controlled trial primarily designed to assess the feasibility and safety of sigmoidoscopy screening,27 and reporting on a combination of incident and prevalent colorectal cancers as outcome, detected by the study’s follow-up colonoscopy.28 Twelve studies were excluded as they did not specifically deal with the effects of screening sigmoidoscopy or colonoscopy.29 30 31 32 33 34 35 36 37 38 39 40 Four articles on randomised controlled trials12 13 14 15 and 12 articles on observational studies1 2 41 42 43 44 45 46 47 48 49 50 were included.
Flow chart of literature search process
Study characteristics of randomised controlled trials
Table 1 provides an overview on design aspects of the four large randomised controlled trials, all of which assessed the impact of screening by flexible sigmoidoscopy on colorectal cancer incidence and mortality. Three studies were from Europe (Norway, United Kingdom, and Italy)12 13 14 and one study was from the United States.15 The numbers of randomised participants ranged from 55 736 in the Norwegian study to 170 432 in the UK trial, but even considerably larger numbers had undergone prescreening for their interest to participate in the UK and Italian trial, whereas the Norwegian study was the only true population based trial. The age range at recruitment was 55-64 in the European trials and 55-74 in the US trial. Participants were enrolled between 1993 and 2001. Median follow-up was 7 years in the Norwegian trial and between 10 and 12 years in the other trials. The three European trials offered once only flexible sigmoidoscopy, which was supplemented by the offer of an additional faecal occult blood test in the Norwegian trial. In the US trial, a second sigmoidoscopy was offered after 3-5 years.
Table 1.
Overview of randomised controlled trials on impact of flexible sigmoidoscopy: design aspects
| Study | Country | No randomised (approached) | Age | Intervention | Years enrolled | Median follow-up (years) | No (%) with lower gastroinestinal endoscopy | Report of per protocol analysis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Adjusted for non-compliance | Adjusted for contamination | |||||||
| Hoff et al 200912 | Norway | 55 736 | 55-64 | Once only FS, with or without single FOBT | 1999-2000 | 7 | 8846/13 653 (64.8) | NR | Yes | No |
| Atkin et al 201013 | United Kingdom | 170 432 (368 142) | 55-64 | Once only FS | 1994-99 | 11.2 | 40 674/57 237 (71.1) | NR | Yes* | No |
| Segnan et al 201114 | Italy | 56 532 (236 536) | 55-64 | Once only FS | 1995-99 | 10.5 | 9999/17 148 (58.3) | NR | Yes* | No |
| Schoen et al 201215 | United States | 154 900 (NR) | 55-74 | FS at baseline and after 3-5 years | 1993-2001 | 11.9 | 67 071/77 445 (86.6) | NR (46.5) | No | No |
FOBT=faecal occult blood test; FS=flexible sigmoidoscopy; NR=not reported.
*With correction for potential differences in outcomes among non-responders and controls.
Participation rates in the intervention group ranged from 58.3% in the Italian trial to 86.6% in the US trial. The high participation rate in the US trial refers to use of at least one screening sigmoidoscopy. Participation rates for individual screening offers at recruitment and after 3-5 years were lower (83.5% and 54.0%, respectively). The US report is the only one that included information on use of lower gastrointestinal endoscopy among controls. Almost half of the controls (46.5%) had any lower gastrointestinal endoscopy during the screening phase (years 0-5), and 48% had a lower gastrointestinal endoscopy after the screening phase. A per protocol analysis, adjusting for non-adherence in the screening group, was reported for the European trials. As per protocol analysis is prone to potential self selection bias, the authors of the UK trial13 and Italian trial14 corrected the per protocol analysis for potential differences in outcomes among non-responders and controls. However, differences were small, and the correction had little impact on point estimates of the per protocol analyses. None of the studies provided effect estimates adjusted for contamination of the control group. Apart from lack of information on and correction for contamination, the quality criteria were mostly fulfilled (see supplementary table 2). Exceptions were the short follow-up of the Norwegian study and non-reporting of per protocol analyses in the US study.
Results of randomised controlled trials
Table 2 summarises the results of the four trials. With relative risks between 0.69 and 0.78 in intention to screen analysis, effect estimates for total colorectal cancer mortality were close in all the trials (even though the estimate failed to reach statistical significance in the Italian trial), suggesting a 22-31% reduction in overall colorectal cancer mortality. Slightly weaker reductions were estimated for total incidence of colorectal cancer, and no reduction was seen in the Norwegian trial. The latter finding most likely results from the shorter follow-up period: in all of the trials it took several years before the initial peak in incidence resulting from identification of prevalent cases at screening was offset by much lower incidence rates in the subsequent years. The pooled estimates (95% confidence intervals) of risk reduction were 18% (11-25%) for incidence of colorectal cancer and 28% (20-35%) for deaths from colorectal cancer (table 2). The per protocol analyses, adjusting for non-adherence in the intervention groups, yielded considerably stronger effect estimates, with pooled estimates for reduction of colorectal cancer incidence and mortality of 28% (16-38%) and 44% (31-54%), respectively.
Table 2.
Overview and meta-analysis of randomised controlled trials on impact of flexible sigmoidoscopy: results on colorectal cancer incidence and mortality. Values are relative risks (95% confidence intervals) unless stated otherwise
| Type of analysis and studies | Incidence | Mortality | |||||
|---|---|---|---|---|---|---|---|
| Any site | Proximal | Distal | Any site | Proximal | Distal | ||
| Intention to screen: | |||||||
| Hoff et al 200912 | 1.02 (0.83 to 1.25)* | NR | NR | 0.73 (0.47 to 1.13) | NR | 0.63 (0.34 to 1.18) | |
| Atkin et al 201013 | 0.77 (0.70 to 0.84) | 0.98 (0.85 to 1.12) | 0.64 (0.57 to 0.72) | 0.69 (0.59 to 0.82) | NR | NR | |
| Segnan et al 201114 | 0.82 (0.69 to 0.96) | 0.91 (0.69 to 1.20) | 0.76 (0.62 to 0.94) | 0.78 (0.56 to 1.08) | 0.85 (0.52 to 1.39) | 0.73 (0.47 to 1.12) | |
| Schoen et al 201215 | 0.79 (0.72 to 0.85) | 0.86 (0.76 to 0.97) | 0.71 (0.64 to 0.80) | 0.74 (0.63 to 0.87) | 0.97 (0.77 to 1.22) | 0.50 (0.38 to 0.64) | |
| Meta-analysis: | |||||||
| No of studies | 4 | 3 | 3 | 4 | 2 | 3 | |
| Pooled estimate | 0.82 (0.75 to 0.89) | 0.91 (0.83 to 0.99) | 0.69 (0.63 to 0.74) | 0.72 (0.65 to 0.80) | 0.95 (0.77 to 1.17) | 0.54 (0.43 to 0.67) | |
| Heterogeneity: I2 (%)/τ2/P value | 52/0.004/0.10 | 0/0.0/0.38 | 24/0.002/0.27 | 0/0.0/0.90 | 0/0.0/0.63 | 0/0.0/0.52 | |
| Per protocol†: | |||||||
| Hoff et al 200912 | 0.89 (0.69 to 1.15)* | NR | 0.73 (0.51 to 1.05)* | 0.41 (0.21 to 0.82) | NR | 0.24 (0.08 to 0.76) | |
| Atkin et al 201013 | 0.67 (0.60 to 0.76) | 0.97 (0.80 to 1.17) | 0.50 (0.42 to 0.59) | 0.57 (0.45 to 0.72) | NR | NR | |
| Segnan et al 201114 | 0.69 (0.56 to 0.86) | 0.85 (0.61 to 1.19) | 0.60 (0.46 to 0.80) | 0.62 (0.40 to 0.96) | 0.78 (0.45 to 1.35) | 0.48 (0.24 to 0.94) | |
| Schoen et al 201215 | NR | NR | NR | NR | NR | NR | |
| Meta-analysis: | |||||||
| No of studies | 3 | 2 | 3 | 3 | 1 | 2 | |
| Pooled estimate | 0.72 (0.62 to 0.84) | 0.94 (0.80 to 1.11) | 0.58 (0.47 to 0.71) | 0.56 (0.46 to 0.69) | 0.78 (0.45 to 1.35) | 0.39 (0.21 to 0.73) | |
| Heterogeneity: I2 (%)/τ2/P value | 49/0.002/0.14 | 0/0.0/0.50 | 49/0.02/0.14 | 0/0.0/0.60 | –/–/1.00 | 6/0.02/0.30 | |
NR=not reported.
P values are based on Cochran’s Q statistic.
*Not reported directly by authors but derived from reported data.
†Adjusted for non-adherence but not for contamination.
Effect estimates were considerably stronger when the analyses were restricted to distal colorectal cancer (table 2). Pooled estimates for reduction in distal colorectal cancer incidence and mortality were 31% (26-37%) and 46% (33-57%), respectively, in intention to screen analysis, and 42% (29-53%) and 61% (27-79%), respectively, in per protocol analyses. Even though point estimates of relative incidence and mortality were also consistently below 1 for cancer ofthe proximal colon in all studies, risk reductions were small and, with the exception of proximal colon cancer incidence in the US trial, not statistically significant, neither in individual studies nor in meta-analysis. Heterogeneity was not evident between studies for any of the mortality outcomes. The apparent heterogeneity in the incidence estimates was due to the Norwegian trial with its shorter follow-up period and was no longer seen when this trial was excluded in sensitivity analyses.
Study characteristics of observational studies
Table 3 provides an overview on the observational studies, which included eight case-control studies and four cohort studies. In one cohort study, participants undergoing screening colonoscopy were compared with the general population46; all other studies compared participants with and without endoscopy. Eight studies were conducted in the United States, and one study each in Canada, Sweden, Switzerland, and Germany.1 2 41 42 43 44 45 46 47 48 49 50 The studies were published between 1992 and 2013 and referred to colorectal cancer diagnoses or deaths between 1970 and 2012, with time frames of endoscopies mostly including 10 or more years before diagnosis or death (or recruitment among controls). One nested case-control study examined the risk for late stage colorectal cancer only (stages IIb to IV).48 All studies were matched or adjusted for age and sex, but other potential confounders were controlled for to a heterogeneous and often limited extent. Apart from lack of or limited control for confounding, lack of assessment of impact of screening on colorectal cancer mortality was the most commonly identified limitation in quality ratings (see supplementary table 3).
Table 3.
Overview on observational studies: design aspects
| Study | Country | Study design | Years (cases) | Lower gastrointestinal endoscopy | Covariates adjusted for or considered | ||
|---|---|---|---|---|---|---|---|
| Reason | Type | Time frame (min, max) | |||||
| Newcomb et al 19921 | United States | Case-control | 1979-88 | Screening | Sigmoidoscopy | bd, ever | Sex, age, family history, duration of enrolment in health plan |
| Selby et al 19922 | United States | Case-control | 1971-87 | Screening | Sigmoidoscopy | bd, 10 years | Sex, age, personal and family history, other screening examinations |
| Scheitel et al 199941 | United States | Case-control | 1970-93 | Screening | Sigmoidoscopy | bd, 10 years | Sex, age, personal and family history, numbers of periodic health examinations and admissions to hospital |
| Slattery et al 200042 | United States | Case-control | NR | Various* | Sigmoidoscopy | 2 years, 12 years | Sex, age, family history, body mass index, physical activity, nutritional factors, acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, HRT (in sensitivity analyses) |
| Newcomb et al 200343 | United States | Case-control | 1998-2002 | Various* | Sigmoidoscopy | 1 year, ever | Sex, age, education, family history, body mass index, smoking, HRT, number of previous tests |
| Cotterchio et al 200544 | Canada | Case-control | 1997-2000 | Various* | Various† | 1 year, ever | Sex, age, marital status, education, family history, medical conditions, body mass index, weight, physical activity, smoking, alcohol consumption, nutritional factors, acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, HRT, other drugs |
| Blom et al 200845 | Sweden | Cohort | 1996-2004 | Screening | Sigmoidoscopy | bd, 8 years | Sex, age |
| Kahi et al 200946 | United States | Cohort‡ | 1989-2007 | Screening | Colonoscopy | bd, 16 years | Sex, age |
| Manser et al 201247 | Switzerland | Cohort | 2001-07 | Screening | Colonoscopy | bd, 7 years | Sex, age, profession, family history, body mass index, physical activity, smoking, nutritional factors, participation in general health screening examinations |
| Doubeni et al 201348 | United States | Case-control | 2006-08 | Various* | Various† | 3 months, 10 years | Sex, age, health plan enrolment, socioeconomic status, comorbidity, family history, other screening exposures |
| Brenner et al 201349 | Germany | Case-control | 2003-10 | Various* | Colonoscopy | 1 year, 10 years | Sex, age, education, family history, body mass index, smoking, acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, HRT, participation in a general health screening examination |
| Nishihara et al 201350 | United States | Cohort | 1988-2012 | Screening | Various† | bd, 25 years | Sex, age, family history, body mass index, physical activity, smoking, alcohol consumption, nutritional factors, acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, HRT, other drugs |
bd=before diagnosis (cases) or reference date (controls); NR=not reported; HRT=hormone replacement therapy.
*Various reasons analysed separately.
†Various types analysed separately.
‡Comparison with general population rather than comparison with unexposed group.
Results of observational studies
Overall, results of the observational studies were more heterogeneous than those of the randomised controlled trials (table 4). Despite the heterogeneity in study designs, settings, populations, and methods, a history of screening sigmoidoscopy or screening colonoscopy was consistently associated with reduced colorectal cancer incidence and mortality in all studies, even though not all results from single studies were statistically significant.
Table 4.
Results and meta-analyses of observational studies on the effects of screening sigmoidoscopy or colonoscopy on colorectal cancer. Values are relative risks (95% confidence intervals) unless stated otherwise
| Study | Type of examination | Incidence | Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Any site | Proximal | Distal | Any site | Proximal | Distal | |||
| Newcomb et al 19921 | Sigmoidoscopy | NR | NR | NR | 0.21 (0.08-0.52) | 0.36 (0.11 to 1.20) | 0.05 (0.01 to 0.43) | |
| Selby et al 19922 | Sigmoidoscopy | NR | NR | NR | NR | 0.96 (0.61 to 1.50) | 0.41 (0.25 to 0.69) | |
| Scheitel et al 199941 | Sigmoidoscopy | NR | NR | NR | 0.89 (0.47-1.66) | 0.95 (0.46 to 1.96) | 1.04 (0.21 to 5.13) | |
| Slattery et al 200042 | Sigmoidoscopy | NR | Men 0.7 (0.5 to 1.1), women 0.5 (0.3 to 0.9) | Men 0.5 (0.3 to 0.7)*, women 0.5 (0.3 to 0.9)* | NR | NR | NR | |
| Newcomb et al 200343 | Sigmoidoscopy | NR | 0.89 (0.68 to 1.16) | 0.24 (0.17 to 0.33) | NR | NR | NR | |
| Cotterchio et al 200544 | Sigmoidoscopy | 0.52 (0.34 to 0.80) | 0.72 (0.51 to 1.01) | 0.41 (0.30 to 0.56) | NR | NR | NR | |
| Blom et al 200845 | Sigmoidoscopy | 0.5 (0.2 to 1.3)† | NR | NR | NR | NR | NR | |
| Doubeni et al 201348 | Sigmoidoscopy | 0.50 (0.36 to 0.70) | 0.79 (0.51 to 1.23) | 0.26 (0.14 to 0.48) | NR | NR | NR | |
| Nishara et al 201350 | Sigmoidoscopy | NR | NR | NR | 0.59 (0.45 to 0.76) | 1.04 (0.73 to 1.48) | 0.31 (0.20 to 0.49) | |
| Cotterchio et al 200544 | Colonoscopy | 0.69 (0.44 to 1.07) | 1.02 (0.72 to 1.45) | 0.68 (0.49 to 0.94) | NR | NR | NR | |
| Kahi et al 200946 | Colonoscopy | 0.52 (0.22 to 0.82) | NR | NR | 0.35 (0.00 to 1.06) | NR | NR | |
| Manser et al 201247 | Colonoscopy | 0.31 (0.16 to 0.59) | NR | NR | 0.12 (0.01 to 0.93) | NR | NR | |
| Doubeni et al 201348 | Colonoscopy | 0.29 (0.15 to 0.58) | 0.36 (0.16 to 0.80) | 0.26 (0.06 to 1.11) | NR | NR | NR | |
| Brenner et al 201349‡ | Colonoscopy | 0.09 (0.07 to 0.13) | 0.22 (0.14 to 0.33) | 0.05 (0.03 to 0.08) | NR | NR | NR | |
| Nishihara et al 201350 | Colonoscopy | NR | NR | NR | 0.32 (0.24 to 0.45) | 0.47 (0.29 to 0.76) | 0.18 (0.10 to 0.31) | |
| Meta-analysis | Sigmoidoscopy | |||||||
| No of estimates | 3 | 5 | 5 | 3 | 4 | 4 | ||
| Pooled estimate | 0.51 (0.39 to 0.65) | 0.76 (0.65 to 0.90) | 0.36 (0.26 to 0.50) | 0.53 (0.30 to 0.97) | 0.96 (0.74 to 1.23) | 0.34 (0.19 to 0.62) | ||
| Heterogeneity: | ||||||||
| I2 (%)/τ2/P value | 0/0.0/0.98 | 0/0.0/0.42 | 64/0.1/0.02 | 68/0.18/0.04 | 0/0.0/0.42 | 54/0.17/0.09 | ||
| Meta-analysis: | Colonoscopy | |||||||
| No of estimates | 5 | 3 | 3 | 3 | 1 | 1 | ||
| Pooled estimate | 0.31 (0.12 to 0.77) | 0.44 (0.15 to 1.31) | 0.21 (0.03 to 1.53) | 0.32 (0.23 to 0.43) | 0.47 (0.29 to 0.76) | 0.18 (0.10 to 0.31) | ||
| Heterogeneity: | ||||||||
| I2 (%)/τ2/P value | 94/1.0/<0.001 | 93/0.86/<0.001 | 97/3.0/<0.001 | 0/0.0/0.69 | —/—/— | —/—/— | ||
| Indirect comparison colonoscopy versus sigmoidoscopy | ||||||||
| No of estimates | 2 | 2 | 2 | 2 | 2 | 2 | ||
| Pooled estimate | 0.61 (0.23 to 1.58) | 0.58 (0.19 to 1.74) | 0.57 (0.07 to 4.32) | 0.59 (0.30 to 1.15) | 0.49 (0.29 to 0.85) | 0.53 (0.23 to 1.21) | ||
| P value | 0.31 | 0.33 | 0.58 | 0.12 | 0.01 | 0.13 | ||
P values are based on Cochran’s Q statistic and, in case of indirect comparison, on significance of log(odds ratio).
NR=not reported.
*Distal colon only.
†Not reported directly by authors, but derived from reported data.
‡Summary results for diagnostic and any colonoscopy, and for distal colorectal cancer not reported in article but obtained by additional analyses.
Meta-analyses of observational studies on screening sigmoidoscopy yielded reductions in colorectal cancer incidence and mortality that were similar to or slightly stronger than those of the per protocol analyses of the randomised controlled trials: a strong reduction in distal colorectal cancer incidence and mortality by 64% (95% confidence interval 50% to 74%) and 66% (38% to 81%), respectively (table 4), weak or no reduction in proximal colon cancer incidence and mortality, and moderate reduction in total colorectal cancer incidence and mortality (table 4). Heterogeneity in the meta-analyses of overall and distal colorectal cancer mortality was mostly due to the study by Newcomb and colleagues,1 a case-control study that had reported an exceptionally strong effect of screening sigmoidoscopy. Excluding this study removed heterogeneity and yielded a slightly less pronounced reduction in mortality (35% and 63% rather than 47% and 66% for overall and distal colorectal cancer mortality, respectively).
Meta-analyses of observational studies on colonoscopy screening consistently yielded stronger reductions in incidence and mortality (table 4). Overall, the incidence of colorectal cancer was estimated to be reduced by 69% (95% confidence interval 23% to 88%). Even though all five studies included in this analysis reported a substantial reduction in colorectal cancer incidence (significantly so in four of the studies), there was substantial heterogeneity in the strength of the estimated reduction of incidence. This heterogeneity was mainly due to the studies by Cotterchio and colleagues from Canada44 and Brenner and colleagues from Germany,49 which showed substantially weaker and stronger risk reductions, respectively, than the remaining studies. Excluding these two studies removed heterogeneity, left the overall estimate of risk reduction essentially unchanged (64%), and strongly increased its precision (95% confidence interval 47% to 75%).
Risk reduction associated with screening colonoscopy was most pronounced for distal colorectal cancer in two out of three studies assessing this association. These two studies, from the United States and Germany, published in 201349 50 are those with the highest quality scores (see supplementary table 3). By contrast, no or only a weak risk reduction was found in the case-control study by Cotterchio and colleagues from Canada,44 which was published in 2005 and pertained to colonoscopies conducted before years 1997-2000, the years that cases were diagnosed. Excluding this study, which also had a lower quality score, from the meta-analysis substantially reduced heterogeneity in relative risk estimates for both proximal and distal colorectal cancer and led to stronger, statistically significant risk reductions (75%, 95% confidence interval 62% to 84%) and 90% (95% confidence interval 52% to 98%), respectively.
Only three studies tackled reduction of mortality associated with screening colonoscopy. They consistently found a strong reduction of overall colorectal cancer mortality, with a summary estimate of 68% (95% confidence interval 57% to 77%). Only the most recently reported cohort study from the United States provided specific estimates for reduction in mortality from proximal and distal colorectal cancer, respectively. Even though mortality reduction was much stronger for distal colorectal cancer (82%, 95% confidence interval 69% to 90%), a substantial and significant mortality reduction was also found for cancer ofthe proximal colon (53%, 24% to 71%). Indirect comparisons of results of observational studies on screening sigmoidoscopy and colonoscopy suggest a 40% to 60% lower risk of colorectal cancer and death from colorectal cancer after screening colonoscopy even though this incremental risk reduction was statistically significant for deaths from cancer ofthe proximal colon only (table 4).
Funnel plots did not suggest relevant publication bias (see supplementary figure 1).
Discussion
This systematic review of randomised controlled trials and observational studies on the impact of lower gastrointestinal endoscopy on colorectal cancer incidence and mortality underlines the strong potential of lower gastrointestinal endoscopy screening to reduce the incidence and mortality from colorectal cancer. Evidence from randomised controlled trials is restricted to screening sigmoidoscopy. The four randomised controlled trials available so far suggest a reduction of total colorectal cancer incidence and mortality by approximately 20-30% in intention to screen analysis and 30-45% in per protocol analyses, with substantially stronger reductions for distal colorectal cancer and no or at best modest reductions for cancer of the proximal colon. Meta-analyses of the identified 10 observational studies on screening sigmoidoscopy suggest similarly strong or even stronger risk reductions than meta-analyses of the randomised controlled trials’ per protocol estimates. Still stronger reductions in colorectal cancer incidence and mortality were seen in the six observational studies on screening colonoscopy, even though the difference between studies on screening sigmoidoscopy and screening colonoscopy was statistically significant for mortality from cancer of the proximal colon only in indirect comparisons.
Comparison with other studies
During the work on this manuscript two meta-analyses exclusively focusing on the randomised controlled trials on screening sigmoidoscopies were published. Despite some differences in inclusion criteria and analyses presented, the main results were consistent.51 52 To our knowledge, ours is the first systematic review and meta-analyses of observational studies. Given the lack of randomised controlled trial results for screening colonoscopy, systematic, joint presentation of results from both types of studies is required to enable the most comprehensive summary of the evidence available to date.
In general, randomised controlled trials are considered the gold standard for estimating screening effects, as they are less prone to several biases than observational studies, such as bias by self selection of screening participants, confounding, and recall bias. It is therefore reassuring that suggestions for the effectiveness of lower gastrointestinal endoscopy screening, which have been raised by multiple case-control and cohort studies since the beginning of the 1990s and which have led to the inclusion of endoscopic examinations in colorectal cancer screening recommendations and offers in several countries, have been consistently supported by results from the four sigmoidoscopy randomised controlled trials that have become available since 2009.12 13 14 15
Comparisons of results from randomised controlled trials and observational studies
A fundamental difference in intention to screen analyses of randomised controlled trials and observational studies on screening is that intention to screen analyses of randomised controlled trials estimate the impact of the offer of screening (irrespective of its actual use), whereas observational studies, like the per protocol analyses of randomised controlled trials, aim to estimate the effects of actual use of screening. The impact of the offer of screening is the variable of primary interest for health policy decisions on implementation of screening offers, whereas the effect of actual use of screening (compared with non-use) is the parameter of primary interest for individual patient consulting and patient decisions. In case of non-negligible non-adherence and contamination, both parameters are necessarily different if screening is effective.
Meta-analyses of observational studies on screening sigmoidoscopy yielded, overall, similar results to the per protocol analyses from the randomised controlled trials. Higher agreement with the per protocol analyses than with the intention to screen analyses is to be expected given that the observational studies, like the per protocol analyses of the randomised controlled trials, deal with the impact of sigmoidoscopies actually performed rather than the impact of the mere offer of screening sigmoidoscopy. Nevertheless, some of the effect estimates from observational studies were even stronger than those of the per protocol analyses from the randomised controlled trials. Several reasons may explain the somewhat stronger effects suggested by the observational studies.
Over-representation of health conscious people who may be more likely to have undergone screening endoscopies among participants in control groups of case-control studies may have led to some overestimation of the prevalence of screening endoscopy and of screening endoscopy effects in observational studies. Furthermore, the possibility of confounding by additional factors, including factors that are of specific concern in screening studies, such as different temporal distribution of screening between cases and controls, or the administering of other screening tests that may have interfered with application of screening endoscopies has to be kept in mind.53
However, even per protocol analyses of randomised controlled trials may have underestimated the impact of screening sigmoidoscopies actually conducted, owing to contamination—that is, the conduction of lower gastrointestinal endoscopies in some proportion of the control group.54 Detailed quantitative data on contamination were provided in the report of the randomised controlled trial from the United States.15 In this trial, contamination was substantial: 46.5% of the controls had either sigmoidoscopy or colonoscopy during the screening phase—that is, the initial 3-5 years, and 48.0% had sigmoidoscopy or colonoscopy after the screening phase. The European trials probably had less contamination owing to less common use of lower gastrointestinal endoscopy in European countries in the past,55 56 but data on the degree of contamination were not provided, and none of the randomised controlled trials accounted for contamination in the analyses.
Other limitations of the randomised controlled trials include the generalisability of results for the population as a whole and for application of screening in routine practice. Finally, the follow-up of the randomised controlled trials may not have been long enough to disclose the full reduction of colorectal cancer incidence. In all the randomised controlled trials there was an initial apparent (and expected) increase in colorectal cancer in the screening group owing to detection of prevalent colorectal cancer, which was compensated by substantially lower incidence in subsequent years. An overall reduction of cumulative incidence was observed after several years only, and this reduction continued to increase during the follow-up periods for which data are available. The apparent lack of reduction of colorectal cancer incidence in the Norwegian trial12 may be primarily due to the shorter follow-up of the participants in this trial compared with the other trials. It remains to be seen in future analyses with longer follow-up times to what extent reduction of incidence will further increase.
In both randomised controlled trials and observational studies on screening sigmoidoscopy the reduction of colorectal cancer incidence and mortality was much stronger or even confined to distal colorectal cancer, which is consistent with expectations given the limited reach of the sigmoidoscope. With a lack of randomised controlled trials and only six observational studies, evidence for the effect of screening colonoscopy is still rather limited, even though it has been substantially strengthened by four most recent studies from the United States, Germany, and Switzerland published in 2012 and 2013.47 48 49 50 Despite some heterogeneity in results, which was to a large extent due to a less rigorous earlier study, meta-analyses suggest a substantially stronger reduction of colorectal cancer incidence and mortality by screening colonoscopy than by screening sigmoidoscopy, in particular for cancer of the proximal colon. Nevertheless, protection from cancer ofthe proximal colon seems to be less pronounced than for distal colorectal cancer even in cases of screening colonoscopy, which might be explained by lower detection rates of proximal compared with distal neoplasms57 58 59 as well as differences in tumour biology.60 61 62 Major differences between proximal and distal cancers have also been found in studies not differentiating between screening and other colonoscopies (which were not included in our review), several of which had not found a protective effect of colonoscopy for proximal cancers.33 34 35 38 Given the still limited data on screening colonoscopy, its impact and relative effectiveness compared with screening sigmoidoscopy should be tackled in further studies. Also, further research is needed in which the incremental effectiveness of screening colonoscopy compared with sigmoidoscopy is weighed against the higher costs, complexity, discomfort, complication rates, and capacities needed compared with screening sigmoidoscopy.63 64 Furthermore, effectiveness of screening colonoscopy may strongly depend on qualification of endoscopists. Performing high quality screening with colonoscopy is challenging and requires major resources in terms of colonoscopy capacity, training, and quality assurance that may not be available or may be difficult to establish in many healthcare systems.
Strengths and limitations of this study
Our review and meta-analysis has several limitations. Firstly, despite a comprehensive literature search in three well established databases independently conducted by two reviewers, and careful cross referencing, we cannot exclude the possibility of having missed a relevant study. Furthermore, we cannot rule out overestimation of screening effects due to publication bias, even though there was no indication of relevant publication bias in the funnel plots. Secondly, there was large heterogeneity in the design of observational studies and in the reporting of results in both randomised controlled trials and observational studies. As a result, the number of studies that could be included in specific meta-analyses was often small. Even though statistical heterogeneity was observed in several of the meta-analyses of the observational studies, especially the studies on screening colonoscopy, results remained essentially unchanged (and heterogeneity was strongly reduced) when excluding the studies with the highest or lowest estimate of risk reduction. Finally, despite inclusion of the most recent studies, the impact was assessed of endoscopies mostly conducted many years if not decades ago. Endoscopies conducted to date might have potentially larger effects due to enhanced detection of neoplasms resulting from advancements in technology, training, and experience of endoscopists.
Conclusions and policy implications
Compelling and consistent evidence from randomised controlled trials and observational studies shows that screening sigmoidoscopy and screening colonoscopy prevent the majority of deaths from distal colorectal cancer. Data suggest added value of colonoscopy versus sigmoidoscopy, especially for prevention of deaths from cancer of the proximal colon, which should be elaborated in further research and weighed against the higher costs, complexity, discomfort, complication rates, and high quality capacities and quality assurance needed,64 65 66 67 as well as possible differences in compliance. To disclose the full benefits of screening sigmoidoscopy, continued follow-up of the large randomised controlled trials will be essential. Future analyses of these trials as well as future analysis of the recently initiated randomised controlled trial on screening colonoscopy16 should pay particular attention to possible underestimation of screening efficacy by non-adherence and contamination. Observational studies should tackle additional issues of major relevance for clinical practice, such as potential variation of risk reduction according to quality of endoscopy, age at endoscopy, or profiles of risk factors (including genetic factors), which might be relevant for risk stratification in colorectal cancer screening.
What is already known on this topic
Observational studies reported since 1992 have suggested a major reduction in incidence of colorectal cancer by screening sigmoidoscopy or screening colonoscopy
Reduction of overall and distal colorectal cancer incidence and mortality by screening sigmoidoscopy was confirmed by four randomised controlled trials published since 2009
In the absence of evidence from randomised controlled trials, which will not be available before the mid 2020s, the added value of screening colonoscopy compared with screening sigmoidoscopy is uncertain
What this study adds
Evidence that screening sigmoidoscopy is able to prevent the majority of deaths from distal colorectal cancer was consistent and compelling
The evidence also suggests substantial added value of screening colonoscopy, especially in the prevention of deaths from cancer of the proximal cancer
Contributors: HB designed the study. MH and CS carried out the literature search. HB and MH extracted the data from the identified articles. CS conducted the meta-analyses. HB drafted the manuscript. All authors critically reviewed the manuscript, contributed to its revision, and approved the final version submitted. The researchers are independent from funders. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported in part by grants from the German Research Council (Deutsche Forschungsgemeinschaft, grant BR 1704/6-4) and the German Federal Ministry of Education and Research (grant No 01ER0814). The sponsors had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the German Research Council and the German Federal Ministry of Education and Research; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Transparency. The lead author (HB, the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Cite this as: BMJ 2014;348:g2467
Web Extra. Extra material supplied by the author
Supplementary file
References
- 1.Newcomb PA, Norfleet RG, Storer BE, Surawicz S, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5. [DOI] [PubMed] [Google Scholar]
- 2.Selby JV, Friedman GD, Quesenberry CP, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992;326:653-7. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber A, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329:1977-81. [DOI] [PubMed] [Google Scholar]
- 4.Stock C, Ihle P, Schubert I, Brenner H. Colonoscopy and fecal occult blood test use in Germany: results from a large insurance-based cohort. Endoscopy 2011;43:771-9. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2012;21:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol 2000;95:868-77. [DOI] [PubMed] [Google Scholar]
- 7.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 2003;124:544-60. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2005. CA Cancer J Clin 2005;55:31-44. [DOI] [PubMed] [Google Scholar]
- 9.Schmiegel W, Pox C, Adler G, Fleig W, Fölsch UR, Frühmorgen P, et al. S3 Guidelines colorectal cancer 2004. [In German.] Z Gastroenterol 2004;42:1129-77. [DOI] [PubMed] [Google Scholar]
- 10.Stock C, Pulte D, Haug U, Brenner H. Subsite-specific colorectal cancer risk in the colorectal endoscopy era. Gastrointest Endosc 2012;75:621-30. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev 2012;21:411-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoff G, Grotmol T, Skovlund E, Bretthauer M, for the Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ 2009;338:b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JMA, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624-33. [DOI] [PubMed] [Google Scholar]
- 14.Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst 2011;103:1310-22. [DOI] [PubMed] [Google Scholar]
- 15.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami H-O, van Ballegooijen M, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy 2012;44:695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA 2000;283:2008-12. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-87. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer G. Meta: Meta-Analysis with R. R package version 3.0-1, 2013. http://CRAN.R-project.org/package=meta.
- 24.Rabeneck L, Paszat LF, Saskin R, Stukel TA. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol 2010;105:1627-32. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertsen VA, Nelms JM. The prevention of invasive cancer of the rectum. Cancer 1978;41:1137-9. [DOI] [PubMed] [Google Scholar]
- 26.Rabeneck L, Lewis JD, Paszat LF, Saskin R, Stukel TA. Risk of proximal and distal colorectal cancer following flexible sigmoidoscopy: a population-based cohort study. Am J Gastroenterol 2008;103:2075-82. [DOI] [PubMed] [Google Scholar]
- 27.Hoff G, Sauar J, Vatn MH, Larsen S, Langmark F, Moen IE, et al. Polypectomy and adenomas in the prevention of colorectal cancer: 10 years’ follow-up of the Telemark Polyp Study I. Scand J Gastroenterol 1996;31:1006-10. [DOI] [PubMed] [Google Scholar]
- 28.Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol 1999;34:414-20. [DOI] [PubMed] [Google Scholar]
- 29.Müller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med 1995;155:1741-8. [DOI] [PubMed] [Google Scholar]
- 30.Müller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. Ann Intern Med 1995;123:904-10. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh AM, Giovannucci EL, Fuchs CS, Colditz GA. Screening endoscopy and risk of colorectal cancer in United States men. Cancer Causes Control 1998;9:455-62. [DOI] [PubMed] [Google Scholar]
- 32.Brenner H, Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G. Long-lasting reduction of risk of colorectal cancer following screening endoscopy. Br J Cancer 2001;85:972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer: a population-based case-control study. Ann Intern Med 2009;150:1-8. [DOI] [PubMed] [Google Scholar]
- 34.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010;139:1128-37. [DOI] [PubMed] [Google Scholar]
- 35.Mulder SA, van Soest EM, Dieleman JP, van Rossum LGM, Ouwendijk RJT, van Leerdam ME, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol 2010;22:437-43. [DOI] [PubMed] [Google Scholar]
- 36.Strock P, Mossong J, Scheiden R, Weber J, Heieck F, Kerschen A. Colorectal cancer incidence is low in patients following a colonoscopy. Dig Liv Dis 2011;43:899-904. [DOI] [PubMed] [Google Scholar]
- 37.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy. A population-based case-control study. Ann Intern Med 2011;154:22-30. [DOI] [PubMed] [Google Scholar]
- 38.Jacob BJ, Moineddin R, Sutradhar R, Baxter NN, Urbach DR. Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc 2012;76:355-64. [DOI] [PubMed] [Google Scholar]
- 39.Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol 2012;30:2664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YR, Cangemi JR, Loftus EV, Picco MF. Risk of colorectal cancer after colonoscopy compared with flexible sigmoidoscopy or no lower endoscopy among older patients in the United States, 1998-2005. Mayo Clin Proc 2013;88:464-70. [DOI] [PubMed] [Google Scholar]
- 41.Scheitel SM, Ahlquist DA, Wollan PC, Hagen PT, Silverstein MD. Colorectal cancer screening: a community case-control study of proctosigmoidoscopy, barium enema radiography, and fecal occult blood test efficacy. Mayo Clin Proc 1999;74:1207-13. [DOI] [PubMed] [Google Scholar]
- 42.Slattery ML, Edwards SL, Ma KN, Friedman GD. Colon cancer screening, lifestyle, and risk of colon cancer. Cancer Causes Control 2000;11:555-63. [DOI] [PubMed] [Google Scholar]
- 43.Newcomb P, Storer BE, Morimoto LM, Templeton A, Potter JD. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst 2003;95:622-5. [DOI] [PubMed] [Google Scholar]
- 44.Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control 2005;16:865-75. [DOI] [PubMed] [Google Scholar]
- 45.Blom J, Yin L, Liden A, Dolk A, Jeppsson B, Pahlman L, et al. A 9-year follow-up study of participants and nonparticipants in sigmoidoscopy screening: importance of self-selection. Cancer Epidemiol Biomarkers Prev 2008;17:1163-8. [DOI] [PubMed] [Google Scholar]
- 46.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770-5. [DOI] [PubMed] [Google Scholar]
- 47.Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening and carcinoma-related death: a closed cohort study. Gastrointest Endosc 2012;76:110-7. [DOI] [PubMed] [Google Scholar]
- 48.Doubeni CA, Weinman S, Adams K, Kamineni A, Buist DSM, Ash AS, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults. Ann Intern Med 2013;158:312-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance or diagnostic colonoscopy. Gastroenterology 2014;146:709-17. [DOI] [PubMed] [Google Scholar]
- 50.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Littlejohn C, Hilton S, MacFarlane GJ, Phull P. Systematic review and meta-analysis of the evidence for flexible sigmoidoscopy as a screening method for the prevention of colorectal cancer. Br J Surg 2012;99:1488-500. [DOI] [PubMed] [Google Scholar]
- 52.Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2012;9:e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss NS, Dhillon PK, Etzioni R. Case-control studies of the efficacy of cancer screening: overcoming bias from non-random patterns of screening. Epidemiology 2004;15:409-13. [DOI] [PubMed] [Google Scholar]
- 54.Brenner H, Stock C, Hoffmeister M. In the era of widespread endoscopy use randomized trials may strongly underestimate effects of colorectal cancer screening. J Clin Epidemiol 2013;66:1144-50. [DOI] [PubMed] [Google Scholar]
- 55.Stock C, Haug U, Brenner H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastroint Endosc 2010;71:366-81. [DOI] [PubMed] [Google Scholar]
- 56.Stock C, Brenner H. Utilization of lower gastrointestinal endoscopy and fecal occult blood test in 11 European countries. Endoscopy 2010;42:546-56. [DOI] [PubMed] [Google Scholar]
- 57.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology 2004;127:452-6. [DOI] [PubMed] [Google Scholar]
- 58.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007;132:96-102. [DOI] [PubMed] [Google Scholar]
- 59.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination. JAMA 2006;295:2366-73. [DOI] [PubMed] [Google Scholar]
- 60.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002;101:403-8. [DOI] [PubMed] [Google Scholar]
- 61.Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 2008;23:418-23. [DOI] [PubMed] [Google Scholar]
- 62.Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010;105:1189-95. [DOI] [PubMed] [Google Scholar]
- 63.Brenner H, Chang-Claude J, Seiler CM, Stürmer T, Hoffmeister M. Potential for colorectal cancer prevention of sigmoidoscopy versus colonoscopy: population-based case control study. Cancer Epidemiol Biomarkers Prev 2007;16:494-9. [DOI] [PubMed] [Google Scholar]
- 64.Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol 2013;108:120-32. [DOI] [PubMed] [Google Scholar]
- 65.Stock C, Ihle P, Sieg A, Schubert I, Hoffmeister M, Brenner H. Adverse events requiring hospitalization within 30 days after outpatient screening and non-screening colonoscopy. Gastrointest Endosc 2013;77:419-29. [DOI] [PubMed] [Google Scholar]
- 66.Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: a population-based analysis. JAMA Intern Med 2013;173:551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro G, Azrak MF, Seeff LC, Royalty J. Outpatient colonoscopy complications in the CDC’s Colorectal Cancer Screening Demonstration Program: a prospective analysis. Cancer 2013;119(Suppl 15):2849-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file