Abstract
We investigated the contribution of the global and the transcription-coupled nucleotide excision repair pathway to the removal of structurally different DNA lesions. The repair kinetics of UV-induced cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone photoproducts (6-4PPs) were determined in an active and inactive gene in normal human fibroblasts and in xeroderma pigmentosum group C (XP-C) fibroblasts. Previously we have shown that in normal human cells exposed to a UV dose of 10 J/m2 repair of CPDs takes place via two pathways: global repair and transcription-coupled repair, the latter being responsible for accelerated repair of CPDs in the transcribed strand of active genes. So far, no clear evidence for transcription-coupled repair of 6-4PPs has been presented. Here we demonstrate that 6-4PPs really form a target for transcription-coupled repair. In XP-C cells, exposed to 30 J/m2 and only capable of performing transcription-coupled repair, CPDs as well as 6-4PPs are removed selectively and with similar kinetics from the transcribed strand of the adenosine deaminase (ADA) gene. The non-transcribed strand of the ADA gene and the inactive 754 gene are hardly repaired. In contrast to XP-C cells, normal cells exposed to 30 J/m2 lack strand-specific repair of both 6-4PPs and CPDs, suggesting that transcription-coupled repair is overruled by global repair, probably due to severe inhibition of transcription at this high UV dose. The much more rapid repair of 6-4PPs compared with CPDs in normal cells may be related to higher affinity of the global repair system for the former lesion. In XP-C cells the similarity of the rate of repair of both 6-4PPs and CPDs in the transcribed strand at 30 J/m2 indicates that transcription-coupled repair of photolesions takes place in a sequential way. Our results strongly suggest that the significance of transcription-coupled repair for removal of lesions depends on the type of lesion and on the dose employed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. DNA methylation. The effect of minor bases on DNA-protein interactions. Biochem J. 1990 Jan 15;265(2):309–320. doi: 10.1042/bj2650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkvens T. M., Gerritsen E. J., Oldenburg M., Breukel C., Wijnen J. T., van Ormondt H., Vossen J. M., van der Eb A. J., Meera Khan P. Severe combined immune deficiency due to a homozygous 3.2-kb deletion spanning the promoter and first exon of the adenosine deaminase gene. Nucleic Acids Res. 1987 Nov 25;15(22):9365–9378. doi: 10.1093/nar/15.22.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Carreau M., Hunting D. Transcription-dependent and independent DNA excision repair pathways in human cells. Mutat Res. 1992 Jun;274(1):57–64. doi: 10.1016/0921-8777(92)90043-3. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., Brouwer J., van de Putte P., McCormick J. J. Preferential repair and strand-specific repair of benzo[a]pyrene diol epoxide adducts in the HPRT gene of diploid human fibroblasts. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5413–5417. doi: 10.1073/pnas.89.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians F. C., Hanawalt P. C. Inhibition of transcription and strand-specific DNA repair by alpha-amanitin in Chinese hamster ovary cells. Mutat Res. 1992 Aug;274(2):93–101. doi: 10.1016/0921-8777(92)90056-9. [DOI] [PubMed] [Google Scholar]
- Donahue B. A., Yin S., Taylor J. S., Reines D., Hanawalt P. C. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R., Sancar A., Reinberg D. Where transcription meets repair. Cell. 1994 Apr 8;77(1):9–12. doi: 10.1016/0092-8674(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Kooiman P., Hessels J. K., Yasui A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem. 1990 May 15;265(14):8009–8015. [PubMed] [Google Scholar]
- Epe B., Müller E., Adam W., Saha-Möller C. R. Photochemical DNA modifications induced by 1,2-dioxetanes. Chem Biol Interact. 1992 Dec;85(2-3):265–281. doi: 10.1016/0009-2797(92)90067-u. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ho L., Bohr V. A., Hanawalt P. C. Demethylation enhances removal of pyrimidine dimers from the overall genome and from specific DNA sequences in Chinese hamster ovary cells. Mol Cell Biol. 1989 Apr;9(4):1594–1603. doi: 10.1128/mcb.9.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas A. L., Vos J. M., Hanawalt P. C. Differential introduction and repair of psoralen photoadducts to DNA in specific human genes. Cancer Res. 1991 Jun 1;51(11):2867–2873. [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993 Mar;12(3):1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattier D. L., States J. C., Hutton J. J., Wiginton D. A. Cell type-specific transcriptional regulation of the human adenosine deaminase gene. Nucleic Acids Res. 1989 Feb 11;17(3):1061–1076. doi: 10.1093/nar/17.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Lawrence D. A. Preferential repair of DNA damage on the transcribed strand of the human metallothionein genes requires RNA polymerase II. Mutat Res. 1991 Jul;255(1):67–78. doi: 10.1016/0921-8777(91)90019-l. [DOI] [PubMed] [Google Scholar]
- McGregor W. G., Chen R. H., Lukash L., Maher V. M., McCormick J. J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nguyen T. D., Cleaver J. E. Nonrandom induction of pyrimidine-pyrimidone (6-4) photoproducts in ultraviolet-irradiated human chromatin. J Biol Chem. 1990 Apr 5;265(10):5353–5356. [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan B., Smerdon M. J. Enhanced DNA repair synthesis in hyperacetylated nucleosomes. J Biol Chem. 1989 Jul 5;264(19):11026–11034. [PubMed] [Google Scholar]
- Ruven H. J., Berg R. J., Seelen C. M., Dekkers J. A., Lohman P. H., Mullenders L. H., van Zeeland A. A. Ultraviolet-induced cyclobutane pyrimidine dimers are selectively removed from transcriptionally active genes in the epidermis of the hairless mouse. Cancer Res. 1993 Apr 1;53(7):1642–1645. [PubMed] [Google Scholar]
- Ruven H. J., Seelen C. M., Lohman P. H., Mullenders L. H., van Zeeland A. A. Efficient synthesis of 32P-labeled single-stranded DNA probes using linear PCR; application of the method for analysis of strand-specific DNA repair. Mutat Res. 1994 Sep;315(2):189–195. doi: 10.1016/0921-8777(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Snyderwine E. G., Bohr V. A. Gene- and strand-specific damage and repair in Chinese hamster ovary cells treated with 4-nitroquinoline 1-oxide. Cancer Res. 1992 Aug 1;52(15):4183–4189. [PubMed] [Google Scholar]
- Tang M. S., Bohr V. A., Zhang X. S., Pierce J., Hanawalt P. C. Quantification of aminofluorene adduct formation and repair in defined DNA sequences in mammalian cells using the UVRABC nuclease. J Biol Chem. 1989 Aug 25;264(24):14455–14462. [PubMed] [Google Scholar]
- Tatsumi K., Toyoda M., Hashimoto T., Furuyama J., Kurihara T., Inoue M., Takebe H. Differential hypersensitivity of xeroderma pigmentosum lymphoblastoid cell lines to ultraviolet light mutagenesis. Carcinogenesis. 1987 Jan;8(1):53–57. doi: 10.1093/carcin/8.1.53. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Okumoto D. S., Sancar A., Bohr V. A. Preferential DNA repair of (6-4) photoproducts in the dihydrofolate reductase gene of Chinese hamster ovary cells. J Biol Chem. 1989 Oct 25;264(30):18005–18010. [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
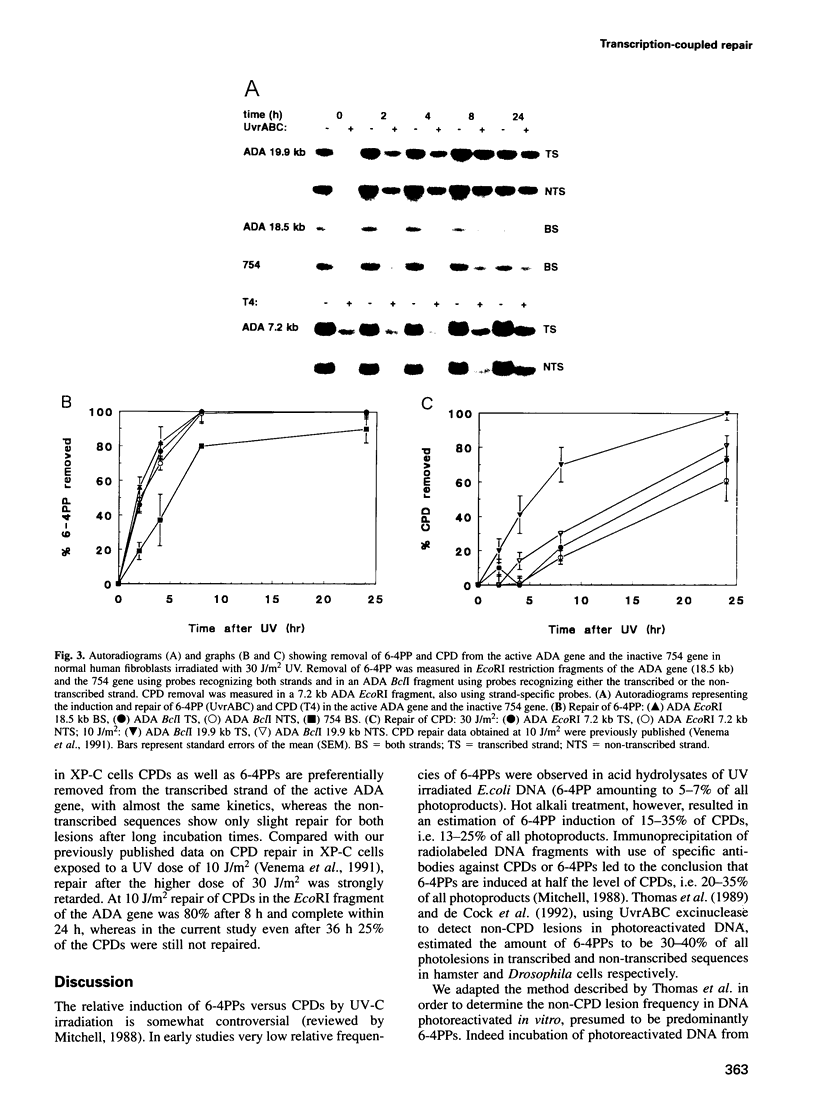
- Venema J., Bartosová Z., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Transcription affects the rate but not the extent of repair of cyclobutane pyrimidine dimers in the human adenosine deaminase gene. J Biol Chem. 1992 May 5;267(13):8852–8856. [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Karcagi V., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Xeroderma pigmentosum complementation group C cells remove pyrimidine dimers selectively from the transcribed strand of active genes. Mol Cell Biol. 1991 Aug;11(8):4128–4134. doi: 10.1128/mcb.11.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Natarajan A. T., van Zeeland A. A., Mullenders L. H. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990 Feb 11;18(3):443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R., de Ruijter M., Moolenaar G. F., van de Putte P. Analysis of UvrABC endonuclease reaction intermediates on cisplatin-damaged DNA using mobility shift gel electrophoresis. J Biol Chem. 1992 Apr 5;267(10):6736–6742. [PubMed] [Google Scholar]
- Vrieling H., Venema J., van Rooyen M. L., van Hoffen A., Menichini P., Zdzienicka M. Z., Simons J. W., Mullenders L. H., van Zeeland A. A. Strand specificity for UV-induced DNA repair and mutations in the Chinese hamster HPRT gene. Nucleic Acids Res. 1991 May 11;19(9):2411–2415. doi: 10.1093/nar/19.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Venema J., Mitchell D. L., van Hoffen A., van Zeeland A. A., Vrieling H., Mullenders L. H., Lohman P. H., Simons J. W. (6-4) photoproducts and not cyclobutane pyrimidine dimers are the main UV-induced mutagenic lesions in Chinese hamster cells. Mutat Res. 1992 Jan;273(1):73–83. doi: 10.1016/0921-8777(92)90051-4. [DOI] [PubMed] [Google Scholar]
- de Cock J. G., van Hoffen A., Wijnands J., Molenaar G., Lohman P. H., Eeken J. C. Repair of UV-induced (6-4)photoproducts measured in individual genes in the Drosophila embryonic Kc cell line. Nucleic Acids Res. 1992 Sep 25;20(18):4789–4793. doi: 10.1093/nar/20.18.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]