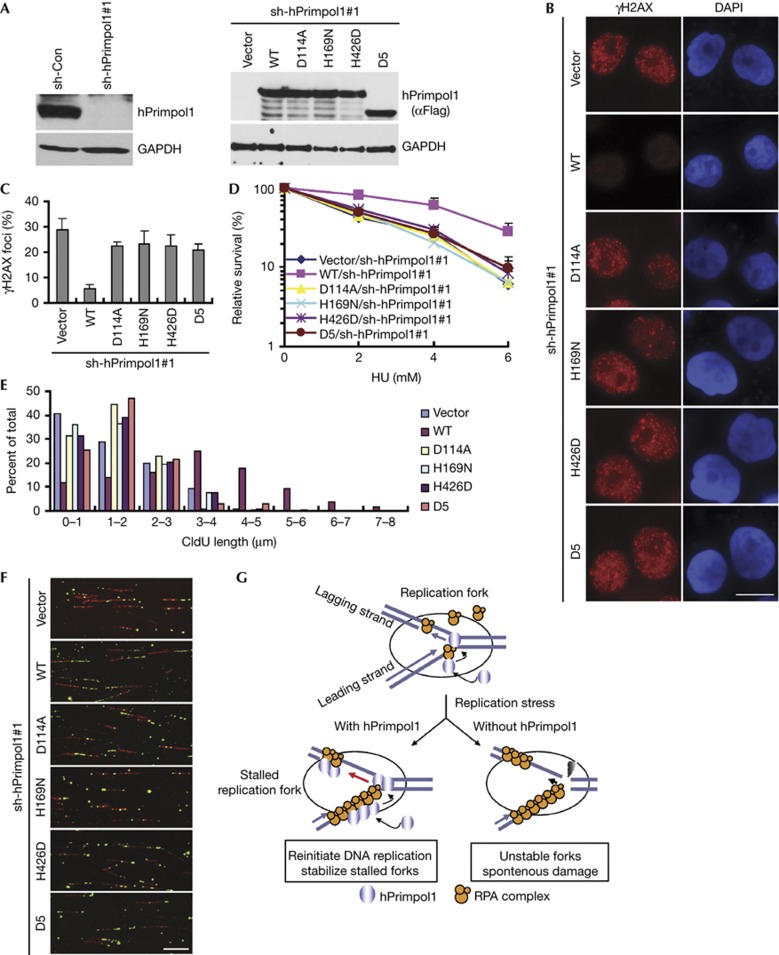
Figure 5.
The RPA1 binding and the primase activity of hPrimpol1 are required for its cellular functions. (A) shRNA-resistant hPrimpol1 and its indicated mutants were transduced into hPrimpol1-deplected HeLa cells. The indicated proteins were analysed in HeLa cells cultured with doxycycline (Dox) for 24 h. (B,C) hPrimpol1 depletion-induced increase of γH2AX foci was rescued by the expression of wild-type hPrimpol1 but not the D5 and the enzyme-inactivating mutants of hPrimpol1. shRNA-resistant hPrimpol1 and its indicated mutants were transduced into hPrimpol1-depleted HeLa cells. Cells were cultured with Dox for 24 h and then subjected to immunostaining using indicated antibodies (B). Scale bar, 10 μm. The quantification of foci-positive cells was performed by counting a total of 200 cells per sample (C). Error bars are s.d.; n=3. (D) Both RPA1 binding and the primase activity of hPrimpol1 are required for restoring cellular resistance to HU. Error bars are s.d.; n=3. (E,F) Defects in fork restart in hPrimpol1-deplected HeLa cells were rescued by wild-type hPrimpol1 but not by its mutants. Quantification of CIdU track lengths was shown (E). Images of DNA fibres isolated from cells treated with hPrimpol1 shRNA following the expression of shRNA-resistant wild-type, the D5, or the enzyme-inactivating mutants of hPrimpol1 (F). Scale bar, 10 μm. (G) A proposed model of hPrimpol1 function at replication forks. Please refer the text for details. hPrimpol1 human primase-polymerase 1; HU, hydroxyurea; shRNA, small hairpin RNA.