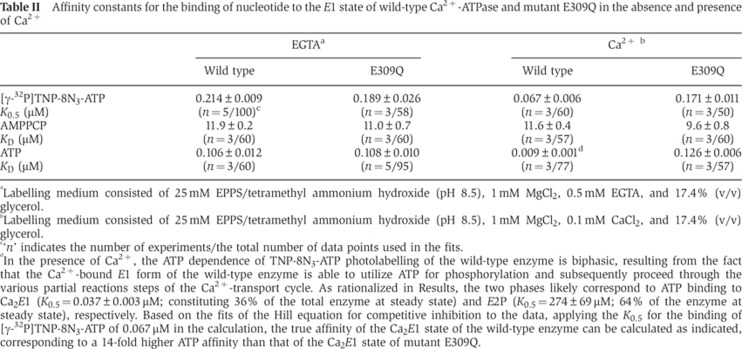
Table II. Affinity constants for the binding of nucleotide to the E1 state of wild-type Ca2+-ATPase and mutant E309Q in the absence and presence of Ca2+.
aLabelling medium consisted of 25 mM EPPS/tetramethyl ammonium hydroxide (pH 8.5), 1 mM MgCl2, 0.5 mM EGTA, and 17.4% (v/v) glycerol.
bLabelling medium consisted of 25 mM EPPS/tetramethyl ammonium hydroxide (pH 8.5), 1 mM MgCl2, 0.1 mM CaCl2, and 17.4% (v/v) glycerol.
c‘n’ indicates the number of experiments/the total number of data points used in the fits.
dIn the presence of Ca2+, the ATP dependence of TNP-8N3-ATP photolabelling of the wild-type enzyme is biphasic, resulting from the fact that the Ca2+-bound E1 form of the wild-type enzyme is able to utilize ATP for phosphorylation and subsequently proceed through the various partial reactions steps of the Ca2+-transport cycle. As rationalized in Results, the two phases likely correspond to ATP binding to Ca2E1 (K0.5=0.037±0.003 μM; constituting 36% of the total enzyme at steady state) and E2P (K0.5=274±69 μM; 64% of the enzyme at steady state), respectively. Based on the fits of the Hill equation for competitive inhibition to the data, applying the K0.5 for the binding of [γ-32P]TNP-8N3-ATP of 0.067 μM in the calculation, the true affinity of the Ca2E1 state of the wild-type enzyme can be calculated as indicated, corresponding to a 14-fold higher ATP affinity than that of the Ca2E1 state of mutant E309Q.