Abstract
Acinar cell carcinoma of the pancreas is a rare pancreatic malignancy, constituting only 1-2% of all the pancreatic tumors. A young adult male presented with chronic diarrhea and upper abdominal pain, on investigations was found to have a large pancreatic tumor of size 12×7.5 cm involving the body of the pancreas. Pancreatic body and tail resection with splenectomy was done and final histopathological examination showed acinar cell carcinoma of the pancreas. Prognosis of acinar cell carcinoma is better than adenocarcinoma of the pancreas.
Key words: pancreatic tumor, resection, positive periodic acid-Schiff stain, acinar cell carcinoma
Introduction
Acinar cell carcinoma, also known as acinic cell carcinoma, is a malignant epithelial neoplasm composed of cells with morphological resemblance to acinar cells and with evidence of exocrine enzyme production by the neoplastic cells. These carcinomas of the pancreas are rarely diagnosed pre-operatively, and surgical resection is the best treatment modality. Median overall survival after successful resection is 36 months in reported studies.1 A brief review of clinical presentation, diagnosis and management is discussed.
Case Report
A 35-year old male patient presented in out patient department, with complaints of diarrhea for last 2 years and pain upper abdomen for last 15 days. There were no other associated symptoms, co-morbidities and he was nonalcoholic and non-smoker. General physical examination revealed no abnormality, and on abdomen examination there was a firm, nontender, 8×8 cm retroperitoneal lump, with well-defined margins, in epigastrc region and also occupying part of left hypochondrial and umbilical region, with no organomegaly and no free fluid. Hematological, biochemical investigations and routine stool examination and Xray chest were normal. Ultrasonography of the abdomen showed a large 7.7×6.4×8.4 cm size heterogeneously hypoechoic mass, involving the body of pancreas, while the head of the pancreas, liver, spleen and other organs were normal. Contrast enhanced computed tomography showed a large, heterogeneously enhancing, well defined intrapancreatic lesion, of size 12×7.5 cm in body and tail region, with few small non-enhancing areas, indentation on posterior wall stomach, and loss of intervening fat plane (Figure 1). There were no evidence of ascites, liver and peritoneal metastasis, and peripancreatic and retroperitoneal lymphadenopathy. Oesopha - gogastroduodenoscopy showed the presence of extrinsic impression on the posterior wall of gastric body. Colonoscopy and tumor marker CA 19-9 were normal. In view of a large mass involving the body of the pancreas and no evidence of metastasis, after adequate preoperative preparation, patient was taken up for surgery. Intra-operatively, there was a large mass of size 12×8 cm arising from the body and part of tail of pancreas, abutting splenic vessels and transverse mesocolon while pancreatic head was normal. The mass was free from stomach, transverse colon, duodenum and spleen (Figure 2), and there were no palpable lymph nodes, liver and peritoneal metastasis and ascites. Distal pancreatectomy with splenectomy was done while the head of the pancreas was preserved. On histopathological examination, grossly, well circumscribed grey brown soft tissue mass measuring 12×8×8 cm, with multinodular external surface, and cut section was variegated with layers of hemorrhage and necrosis, and on microscopy, there was partly encapsulated tumor composed of cells arranged mainly in diffuse sheets, trabecular and focally in acinar pattern, at places tumor sheets are separated by thick fibrous septa and cells shows mild pleomorphism, round in shape, with indistinct cell membranes, moderate amount of granular eosinophilic cytoplasm, round to oval hyperchromatic nuclei (Figure 3A). Periodic acid-Schiff (PAS) staining with diastase showed granular cytoplasmic positivity (Figure 3B), and immunohistochemistry demonstrated acinar enzymes, trypsin and chymotrypsin. This histopathological picture was suggestive of acinar cell carcinoma of the pancreas. Post-operative period was uneventful and the patient received 6 cycles of chemotherapy (FOLFOX regimen) and got discharged on post-operative day 18.
Figure 1.
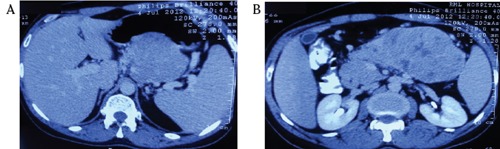
A) and B) Contrast enhanced computed tomography showing a large, heterogeneously enhancing, well defined intrapancreatic lesion, of size 12×7.5 cm in body and tail region, with few small non-enhancing areas, indentation on posterior wall stomach, and loss of intervening fat plane.
Figure 2.
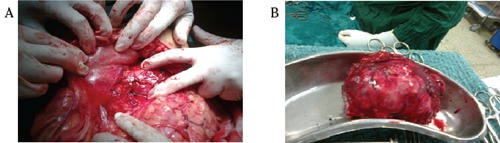
A) and B) Intraoperative pictures showing a large tumor mass in the body of pancreas, stomach, transverse colon and head of pancreas.
Figure 3.
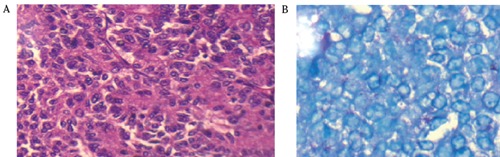
A) Partly encapsulated tumor composed of cells arranged mainly in diffuse sheets trabecular and focally in acinar pattern; B) Periodic acid-Schiff staining with diastase showing granular cytoplasmatic positivity.
Discussion
Acinar cell carcinoma is a rare pancreatic malignancy, and constitutes 1-2% of all exocrine pancreatic neoplasms in adults and 15% of those in pediatric age group of all pancreatic tumors.2-4 Though acinar cells makeup the bulk of the pancreas, pancreatic neoplasms exhibiting predominantly acinar differentiation are rare. Not more than 25% of the neoplastic cells exhibit endocrine or ductal components and cases with significant endocrine or ductal components (more than 25%) are regarded as mixed carcinomas. There is a slight male preponderance and mostly presents in 5th to 7th decades of life. Acinar cell carcinomas arise more commonly from head though they may arise from any part of the pancreas. Most commonly, acinar cell carcinomas present with non-specific symptoms like weight loss (52%), abdominal pain (32%), nausea and vomiting (20%), diarrhea (8%), weakness (12%), anorexia (12%). Patients might present with an asymptomatic lump. In contrast to ductal adenocarcinoma, acinar cell carcinoma rarely obstructs the common bile duct because they generally compress and do not infiltrate into adjacent structures, so jaundice is infrequent (12%).4 Our patient presented with chronic diarrhea and upper abdominal pain.
They have a unique ability to produce pancreatic enzymes. Paraneoplastic syndrome may be the only presenting symptom in 15% of patients, and lipase hypersecretion syndrome 4,5 characterized by subcutaneous fat necrosis, polyarthralgia and peripheral eosinophilia is a type of paraneoplastic syndrome associated with it. This syndrome is more commonly encountered in patients with hepatic metastasis, although occasionally this could be due to an extremely large organ-limited primary carcinoma. 2-4 Successful surgical removal of the neoplasm may result in the normalization of the serum lipase levels and resolution of the symptoms. Serum - fetoprotein level may be elevated in some cases.6 Serum glycoprotein markers (CA 19-9) are usually not elevated and a modest elevation in serum lipase levels may be detected in those patients without the lipase hypersecretion syndrome.
Hoorens et al. analyzed cell lineage markers, p53 expression, and K-ras mutations of acinar cell carcinoma, demonstrating that they constitute an entity different from ductal adenocarcinoma or endocrine tumors.3 In recent molecular analysis of acinar cell carcinomas, it was found that there are abnormalities in the APC/βcatenin pathway similar to those found in colorectal cancer.7 For this reason, chemotherapeutic agents effective in pancreatic adenocarcinoma and colorectal carcinoma like gemcitabine, cisplatin, 5FU, leucovorin, oxaloplatin, irinotecan, capecitabine etc. are effective in acinar cell carcinoma,8 and we also gave our patient FOLFOX regimen. Acinar cell carcinomas are highly cellular tumors with minimal stroma and lack stromal desmoplasia. Four patterns of growth have been described: acinar, cellular, trabecular, and glandular. In our case, it was mixed acinar and trabecular pattern. PAS positive, diastase resistant cytoplasmic granules are commonly present in tumor cells.9 Acinar enzymes, especially trypsin, chymotrypsin are demonstrable by immunohistochemistry in essentially all cases.
In most of the studies, approximately 50% cases presented with metastasis at the time of diagnosis and the most common site of metastasis was liver. Surgical resection is the best treatment for localized acinar cell carcinomas. It is different from adenocarcinoma in that it has more indolent course with 5-year survival over 40%, with median survival 36 month, and the genetic alteration of ductal adenocarcinoma like KRAS, TP53, P16, or SMAD4 are absent in acinar cell carcinomas.3,4
In conclusion, acinar cell carcinomas are rare pancreatic tumors, present mostly with non-specific symptoms, difficult to diagnose pre-operatively, surgery is the mainstay of therapy, and there are no standard chemotherapy regimens and prognosis is better than adenocarcinomas.
References
- 1.Adsay NV, Kimastra DS.Pancreatic and periampullary tumors: classification and pathologic features. Jarnagin WR, Blumgart’s surgery of the liver, biliary tract, and pancreas. 5th edition Philadelphia, PA: Saunders-Elsevier; 2012. p 981 [Google Scholar]
- 2.Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of pancreas and related tumors. J Clin Oncol. 2002; 20:4673-8 [DOI] [PubMed] [Google Scholar]
- 3.Hoorens A, Lemoine NR, McLellan E, et al. Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and Ki-ras mutation. Am J Pathol. 1993; 143:685-98 [PMC free article] [PubMed] [Google Scholar]
- 4.Klimsatra DS, Heffess CS, Oertel JE, Rosai J.Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992; 16:815-37 [DOI] [PubMed] [Google Scholar]
- 5.Klimstra DS, Adsay NV.Acinar cell carcinoma of the pancreas. A case associated with the lipase hypersecretion syndrome. Pathol Case Rev. 2001; 6:121-6 [Google Scholar]
- 6.Mueller SB, Micke O, Herbst H, et al. Alpha-fetoprotein-positive carcinoma of the pancreas: a case report. Anticancer Res. 2005; 25:1671-4 [PubMed] [Google Scholar]
- 7.Abraham SC, Wu T, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma. Am J Pathol. 2002; 160:953-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royal RE, Wolf RA, Crane CH.Pancreatic cancer. DeVita VT, Lawrence TS, et al., DeVita, Hellman and Rotenberg cancer principle & practice of oncology. 8th edition Philadephia, PA: Lippincott; 2011. p 1118 [Google Scholar]
- 9.Camp ER, Tamm EP, Gomez HF, et al. Unusual pancreatic tumors. Yeo CJ, Shackelford’s surgery of the alimentary tract. 6th edition Philadelphia, PA: Saunders-Elsevier; 2005. pp 1432-1434 [Google Scholar]


