Figure 2.
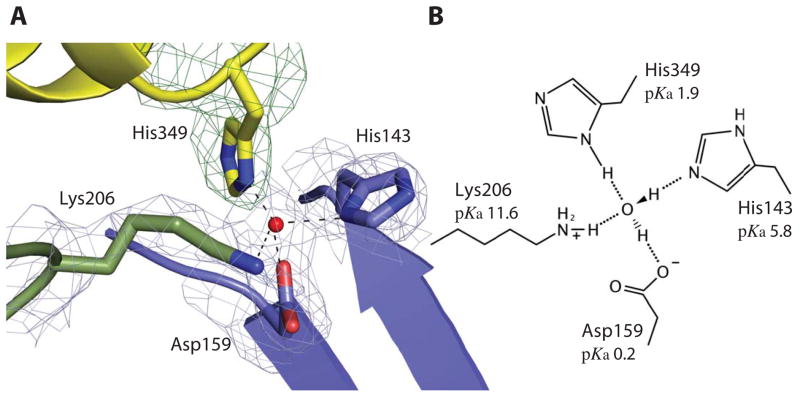
TbpB stabilizes the holo form of hTf. A The cartoon representation of the buried His349 from hTf in contact with His143, Asp159, Lys206 from TbpB illustrates their interaction through a tetra-coordinated bridging water within the binding interface. The four residues and the tetrahedral coordinated water (red sphere) are embedded within a simulated annealing 2Fo-Fc electron density map at 1.0 sigma missing the hTf His349 residue. Domains colored as in figure 1a (cyan, yellow and blue denote the C1 and C2 hTf domains and the N-lobe TbpB handle domain, respectively). B The schematic model of the tetra-coordinated water is shown with structure-based predicted pKa values for each residue indicated.