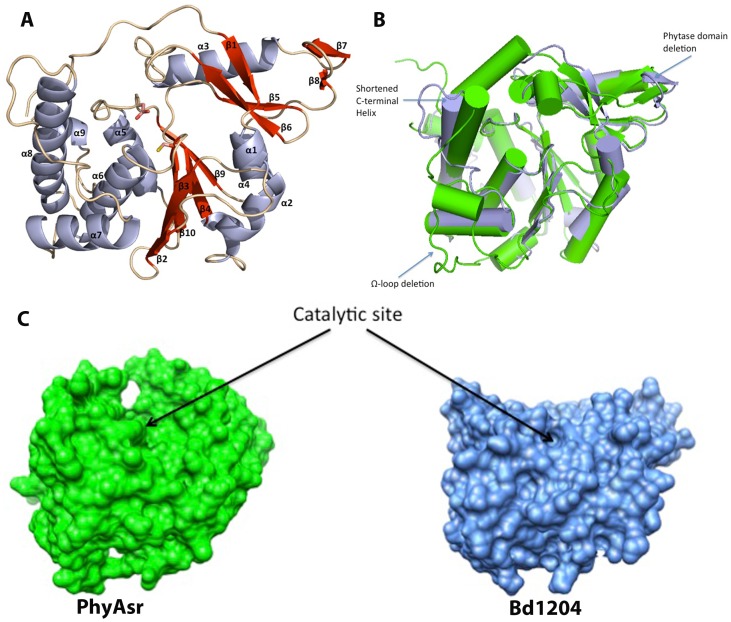
Figure 2. Structure of Bd1204 and comparison to known phytases.
A) Bd1204 fold. The phytase domain is composed of strands β1, 5, 6, 7, 8 and helix α3. The catalytic nucleophile (C206) and general acid (D177) are shown as sticks. The phosphate binding loop is located at the center of the active site between β10 and α6. B) Least squares superposition of Bd1204 (light blue) on the Selenomonas ruminantium phytase (Green). Significant deletions observed in Bd1204 in the Phytase domain and the Ω-loop are Highlighted. C) Comparison of the surface of the active sites in PhyAsr (Green) and Bd1204. Structures are rotated so that the bottom of the central β-sheet is pointing out of the plane of the page and the phytase domain is into the plane of the page. The surface of Bd1204 is much flatter which results in a much shallower active site relative to PhyAsr.