Abstract
This study examined the association between prenatal cocaine exposure (PCE) and developmental trajectories of externalizing behavior problems from 18 to 54 months of child age. A hypothesized indirect association between PCE and externalizing trajectories via maternal negative affect was also examined. Caregiving environmental risk and child sex were evaluated as moderators. This study consisted of 196 mother-child dyads recruited at delivery from local area hospitals (107 cocaine exposed, 89 non-exposed) and assessed at 7 time points across the toddler to preschool periods. Results revealed no direct associations between PCE and externalizing behavior problem trajectories. However, results did indicate that PCE shared a significant indirect relationship with externalizing behavior problem trajectories via higher levels of maternal negative affect. The association between PCE and externalizing problem trajectories was also moderated by caregiving environmental risk such that PCE children in high-risk caregiving environments did not experience the well documented normative decline in externalizing behavior problems beginning at around three years of age. This study suggests potential pathways to externalizing behavior problems among high-risk children.
Keywords: Cocaine, Behavior Problems, Prenatal Substance Exposure, Trajectory, Externalizing
Introduction
Prenatal cocaine exposure (PCE) has the potential to disrupt monoaminergically regulated systems implicated in regulation of affect and behavior, such as the mesolimbic pathway as well as dopaminergic pathways associated with attentional systems, such as the striatal-prefrontal pathway (Gawin & Ellenwood, 1988; Nassogne, Evrard, & Courtoy, 1998; Li et al., 2011; Liu & Lester, 2011; Mayes, 2002). However, PCE studies assessing behavioural outcomes related to regulation of affect and behaviour such as externalizing behavior problems have yielded mixed findings in early childhood. Some studies, for instance, have found that PCE was directly related to child externalizing behavior problems (e.g., Richardson, Goldschmidt, & Wilford, 2009), some have found that PCE was only associated with behavior problems for boys (Bailey et al., 2005; Delaney-Black et al., 2004), and still others have reported no direct relationship between PCE and child externalizing behavior problems (e.g., Accornero et al., 2002; Bennett, Bendersky, & Lewis, 2002; Warner et al., 2006).
While the aforementioned studies examined the association between PCE and externalizing behavior problems over time, they did not assess the developmental trajectories of externalizing behavior problems. Examining developmental trajectories of externalizing behavior problems has advantages over studying externalizing problems at discrete periods of time. Most importantly, studying developmental trajectories allows one to examine changes in both the level and the rates of growth or decline of these behaviors over time, putting developmental changes in externalizing behaviors at the forefront (Nagin, 1999). Indeed, research has demonstrated that the overall developmental trajectory of externalizing behavior problems from 18 to 54 months is nonlinear, peaking at three years of age and declining thereafter (Campbell, 1995; Cummings, Iannotti, & Zahn-Waxler, 1989; Nagin & Tremblay, 1999). However, there is a small group of children who are persistently aggressive or defiant, do not exhibit the normative declines in externalizing problems, and are at high risk for later psychopathology (Campbell, 1995; Nagin & Tremblay, 1999). Thus, examining developmental trajectories over a period of normative change may be particularly informative.
The few studies that have specifically examined developmental trajectories of externalizing behavior problems among PCE children while accounting for other prenatal substance use have also produced conflicting findings. Bada et al. (2007), for example, found that high levels of PCE had a direct negative impact on externalizing behavior problem trajectories among children aged three to five years. Minnes et al. (2010), on the other hand, found that PCE was only related to behavior problem trajectories for delinquency and that this association was only present among girls aged four to ten years. Finally, among children aged two and a half to five and a half years, Chaplin, Fahy, Sinha, and Mayes (2009) found that PCE was not a significant predictor of trajectories of externalizing behavior problems.
One factor that needs to be considered when evaluating these mixed findings is the strength of the association or the magnitude of the effect (i.e., effect size, APA, 2001, p.25). For instance, while Bada et al. (2007) did find a direct link between PCE and trajectories of externalizing behavior problems, they found that this association was quite modest in terms of effect size despite their relatively large sample size. Thus, it is possible that large sample sizes are required to identify subtle effects associated with PCE. However, one could also argue that for an outcome such as externalizing behavior problems, small effect sizes associated with PCE in early childhood may not be clinically meaningful.
However, another plausible explanation for these equivocal findings may be the presence of moderating or mediating factors. A developmental psychopathology perspective acknowledges that different influences contribute to the onset and maintenance of behavior and researchers from this tradition have long promoted the study of mediators and moderators of child outcomes among high-risk cohorts (Cicchetti & Toth, 2009; Rutter & Garmezy, 1983). With regard to mediation, both theory and empirical evidence suggest a number of pathways by which PCE may impact child externalizing behavior problems. One of these potential pathways is through maternal parenting behavior. Animal studies indicate dose-dependent associations between cocaine treatment during gestation and disruptions in maternal caretaking behavior in the immediate post-partum period, perhaps due to decreased oxytocin levels (Johns et al., 1998, 2005; Nelson et al., 1998; Vernotica, Lisciotto, Rosenblatt, & Morrell, 1996). While maternal parenting behavior is not typically assessed as a mediator of outcome among human PCE samples, it is a salient, proximal predictor of externalizing behavior problems across multiple low and high risk samples of children (Bradley & Corwyn, 2007; Erath, El-Sheikh, Hinnant, & Cummings, 2011; Miner & Clarke-Stewart, 2008; Olson et al., 2011; Stormshak, Bierman, McMahon, Lengua, & Conduct Problems Prevention Research Group, 2000). Moreover, studies have demonstrated that maternal parenting behavior varies as a function of maternal substance use. Although maternal cocaine use during pregnancy is not universally associated with lower quality parenting, cocaine using mothers are at higher risk for being disengaged and passive during mother-infant interactions in the neonatal period (Gottwald & Thurman, 1994); are less flexible and engaged during feeding interactions (LaGasse et al., 2003); have lower responsiveness and enthusiasm in later infancy (Burns, Chethik, Burns, & Clark, 1997); are less emotionally engaged in the toddler period (Molitor, Mayes, & Ward, 2003); use fewer positive reinforcements and more threats of physical discipline in the toddler/preschool period (Bauman & Dougherty, 1983); display more harshness during different laboratory based interactions at 2 years of age (Eiden, Schuetze, Colder, & Veira, 2011); and are more hostile and intrusive in a structured teaching situation at 3 years of age (Johnson et al., 2002).
One common theme across these dimensions of parenting is the presence or absence of negative affect defined as anger, hostility, and displeasure expressed during mother-child interactions and also referred to in the literature as harshness or hostility. In addition to associations with maternal substance use including cocaine, this dimension of parenting behavior has been most consistently associated with externalizing behavior problems (Bradley & Corwyn, 2007; Erath et al., 2011; Olson et al., 2011; Rubin et al., 1998; Rubin et al., 2003; Stormshak et al., 2000), including PCE samples (Bennett et al., 2002). The association between maternal negative affect and child behavior problems may reflect modeling of poor self-control, some aspect of genetic risk, or a combination of both. Taken together, these findings suggest that maternal negative affect toward the child may function as a mediator of the association between PCE and child externalizing behavior problems in the toddler period.
While mediation assesses potential pathways to risk or resilience, moderation tests whether particular variables exacerbate or ameliorate risk or resilience. Recent studies of PCE indicate that child sex and environmental risk may be important moderators of the relationship between PCE and child externalizing behavior problems. Findings from both animal and human studies, for instance, suggest that boys may be more susceptible than girls to the effects of PCE in some aspects relevant to the development of externalizing behavior problems, such as selective attention, sustained attention, and greater affective reactivity (Beaudin, Gendle, & Strupp, 2011). Moreover, recent studies have reported significant relationships between PCE or heavy cocaine exposure and child externalizing behavior problems for boys, but not for girls at school age (Bailey et al., 2005; Delaney-Black et al., 2004). Contrary to these findings; however, are studies which have either demonstrated that the association between PCE and child behavior problems does not vary as a function of child sex (Bada et al., 2011) or is only present among girls (Minnes et al., 2010). Thus, while child sex is an important consideration when examining the relationship between PCE and externalizing behavior problems, the nature of its association remains unclear.
In addition to child sex, numerous studies that have failed to detect a direct association between PCE and behavior problems have underscored the importance of the postnatal environment (Bennett, Bendersky, & Lewis, 2007; Brown, Bakeman, Claire, Platzman, & Lynch, 2004; Singer et al., 2004). PCE children, for instance, are more likely to experience higher levels of environmental risk or caregiving instability as indicated by a number of factors such as: frequent separations from the primary caregiver, changes in caregiving adults, frequent changes in the living situation, and lack of a male caregiver (Brown et al., 2004). Maternal cocaine use has also been associated with higher levels of psychiatric symptoms across a number of studies (Bendersky, Alessandri, Gilbert, & Lewis, 1996; Eiden, Foote, & Schuetze, 2007; Woods, Eyler, Behnke, & Conlon, 1993), and evidence clearly links higher rates of maternal psychopathology to higher rates of externalizing behavior problems (Campbell, Pierce, Moore, Marakovitz, & Newby, 1996; Gartstein, Bridgett, Dishion, & Kaufman, 2009). Finally, accumulating evidence indicates that substance-using women may be more likely to place their children at risk for exposure to violence (Conner-Burrow, Johnson, & Whiteside-Mansell, 2009; Bada et al., 2011).
Empirical evidence and theoretical discussions on the impact of risk and protective factors on a number of developmental outcomes have noted that multiple risk conditions often co-occur (Pungello, Kupersmidt, Burchinal, & Patterson, 1996; Rutter, 1987; Sameroff, Seifer, Baldwin, & Baldwin, 1993; Seifer, 1995; Zeanah, Boris, & Larrieu, 1997). Given this situation, researchers have argued that the total number of environmental risk factors may be more predictive of child outcomes than exposure to any specific risk condition (Seifer, 1995; Zeanah et al., 1997). There is a great deal of heterogeneity in the caregiving situations of cocaine exposed children (Brown et al., 2004; Eiden et al., 2007). Consequently, it is important to examine the role of cumulative caregiving risk as a moderator of the association between PCE and child behavior problems. Furthermore, according to diathesis-stress models one would indeed expect the joint influence of prenatal substance exposure and high levels of caregiving environmental risk to be associated with the poorest outcomes.
The prevalence of polydrug use among cocaine using mothers must also be taken into account. Cocaine-using mothers commonly use other substances such as marijuana, cigarettes, and alcohol, and findings from both animal and human studies haves shown that prenatal cigarette and alcohol exposure may have significant effects on child externalizing behavior problems (for review, see Dixon, Kurtz, & Chin, 2008; Wakschlag & Hans, 2002). Therefore, the link between PCE and developmental trajectories of externalizing behavior problems must be investigated in the context of comorbid substance use.
In sum, the aim of this study was to examine direct and indirect associations between PCE and developmental trajectories of externalizing behavior problems from 18 to 54 months of age, while accounting for the effects of polydrug use. Moreover, we tested whether maternal negative affect during mother-infant interactions mediated the association between PCE and developmental trajectories of externalizing behavior problems. Finally, we assessed whether child sex and cumulative caregiving environmental risk moderated the above associations.
Based on the literature, several hypotheses were tested in the present study. First, we hypothesized that there would be individual differences or significant variability in the developmental trajectory of externalizing behavior problems from 18 to 54 months. Second, we expected that the overall developmental trajectory of externalizing behavior problems from 18 to 54 months would be nonlinear, peak at three years of age and decline thereafter (Campbell, 1995; Cummings et al., 1989; Nagin & Tremblay, 1999). Third, we hypothesized that PCE toddlers would have higher levels of externalizing behavior problems at 18 months (time 1), would exhibit an increasing rate of change in externalizing behavior problems from 18 to 36 months, and would not demonstrate the same level of decline from 36 to 48 months of age relative to non-PCE toddlers. Fourth, we expected that maternal negative affect would mediate the association between PCE and developmental trajectories of externalizing behavior problems. Specifically, we postulated that PCE toddlers would experience higher levels of maternal negative affect. This, in turn, would be related to higher initial levels of externalizing behavior problems, to a greater increase in rate of change in externalizing behavior problems from 18 to 36 months of age, and to lower levels of decline from 36 to 48 months of age. Fifth, we hypothesized that the impact of PCE on developmental trajectories of externalizing behavior problems would vary by cumulative caregiving environmental risk, such that the combination of PCE and caregiving environmental risk would be associated with the highest initial levels of externalizing behavior problems, a higher rate of increase in externalizing problems, and less of a normative decline compared to those experiencing no PCE and low environmental risk. Finally, we also tested whether child sex moderated the above associations, but had no specific hypotheses given that the findings have been mixed.
Methods
The sample consisted of 196 mother-child dyads participating in an ongoing longitudinal study of PCE, (107 PCE, 89 NCE). An outreach worker on the project staff recruited all participants after delivery from two local area hospitals. Mothers ranged in age from 18 to 42 years (M =29.65, SD =6.04). The majority of the mothers were African American (74%), were receiving Temporary Assistance for Needy Families (71%) at the time of their first laboratory visit (years 2001–2004), and were single (60%). Of the 196 children, 97 (49.5%) were boys. All families were recruited from two local area hospitals serving a predominantly low-income population and the two groups were matched on maternal education, maternal race/ethnicity, and infant sex. 11% of the PCE infants (ranged from 32 to 41 weeks) and 3% of the NCE infants (ranged from 36 to 41 weeks) were preterm (i.e., < 37 weeks gestational age). Infants ranged from 1531 to 5072g at birth (M = 3113.38, SD = 558.64). The study received approval from the institutional review boards of the hospitals as well as the primary institution at which the study was conducted. Informed written consents were obtained from all recruited participants. Participants were compensated for their time in the form of gift certificates, checks, and child toys at each assessment, with the amount increasing over time.
Procedure
All mothers were screened using a general health and substance use screener after delivery for initial eligibility and matching criteria. The inclusion criteria for the PCE group were that the infants had to have experienced prenatal cocaine exposure (details on how this was assessed are below). Exclusionary criteria for all mothers were (a) maternal age less than 18 years, (b) use of illicit substances other than cocaine or marijuana during pregnancy, and (c) significant medical problems in the infant (e.g., congenital anomalies, FASD diagnosis, HIV+ status, genetic disorders, baby in critical care for over 48 hours, and prolonged respiratory distress). Interested and eligible mothers were given detailed information about the study by the recruiter and asked to sign consent forms. About 2 weeks after delivery, mothers were contacted and scheduled for their first laboratory visit, which took place at the time that their infant was approximately 4–8 weeks old. Additional assessments were scheduled when the child was 7, 13, 18, 24, 30, 36, 42, 48, and 54 months. Assessments at 4–8 weeks, 7, 13, 24, 36, and 48 months consisted of a combination of maternal interviews, observations of mother-child interactions, and child assessments. Assessments at 18, 30, and 42 months consisted of maternal interviews only. Regardless of care situation, biological mothers were interviewed at the 4- to 8-week assessment in addition to the primary caregiver (if different) to obtain accurate information about prenatal substance use. In the circumstances of a change in custody arrangements, the person who had legal guardianship of the child was also contacted and asked to participate.
Once a family was recruited into the cocaine group, the closest matching comparison family was recruited (i.e., matched on maternal education, maternal race/ethnicity, and infant sex). However, a significantly higher proportion of mothers in the comparison group declined participation or withdrew before formal enrollment, resulting in a smaller number of families in the control group. Of the 4,800 women screened at delivery, 340 were eligible for participation in either group. Of these 340 women, 35% either declined participation or were not enrolled in the study because they expressed initial interest but later withdrew or did not keep their appointment within the narrow window of child assessment time, resulting in a sample of 220 mother-infant dyads. Of these 220 mother-infant dyads, 4 were excluded from analyses (two infants were later diagnosed with fetal alcohol syndrome, one was later diagnosed with shaken baby syndrome, one infant was severely delayed) resulting in 216 eligible families. Of these, twenty did not have data on child externalizing behavior problems at any of the time points), resulting in a final sample of 196 dyads. Our retention rates at 7, 13, 18, 24, 30, 36, 42, 48, and 54 months, have been 87%, 87%, 83%, 82%, 83%, 77%, 81%, 76%, and 79% of the 216 families. Mothers who participated were more likely to be between 18 and 25 years of age, (p < .001), and were more likely to have a high school or below high school education (p < .001), compared to those who were eligible but not enrolled. Mothers who participated were also more likely to be in the cocaine group (with a participation rate of 91% among cocaine group eligible) compared to those who were eligible but not enrolled. The majority of mothers in the cocaine group who were eligible but not enrolled in the study had children who were placed in non-maternal care. There were no other differences on any demographic variables between those who participated and those who were eligible but not enrolled or between mothers in the cocaine group who participated compared to those who did not.
Assessment of growth and risk status
Three measures of growth were used in this study: birth weight (gm), birth length (cm), and head circumference (cm). All measurements were taken by obstetrical nurses in the delivery room and recorded in the infant’s medical chart. Research staff recorded this information from the charts after recruiting the mother-infant dyad. Medical chart review at the time of recruitment also was used to complete the Obstetrical Complications Scale (OCS; Littman and Parmelee, 1978), a scale designed to assess the number of perinatal risk factors experienced by the infant. Higher numbers on this scale indicate a more optimal obstetric score. Gestational age was calculated by dates and extracted from medical records.
Identification of substance use
Cocaine status was determined by a combination of maternal report, chart review, maternal hair and urine analysis. Urine toxicologies were routinely conducted at the first prenatal visit on maternal urine and/or at delivery (for those mothers who tested positively prenatally, obtained prenatal care elsewhere, or did not receive any prenatal care) on infant and maternal urine by participating hospitals. All mothers and children in both the exposed and non-exposed groups received a biological assay in the newborn period to determine or rule out PCE. Mothers were included in the cocaine group if self-reports were positive, regardless of urine toxicology or hair sample results. Similarly, mothers who reported that they did not use cocaine but had positive urine toxicology or hair samples were included in the cocaine group.
Urine toxicology consisted of standard urine screening from drug level or metabolities of cocaine, opiates, benzodiazepines, and tetrahydrocannabinol. Urine was rated positive if the quantity of drug or metabolite was >300g/ml. Hair samples were collected from the mothers at the first laboratory visit and sent to Psychemedics Corporation for Radioimmunoanalyses (RIAH). RIAH is the most well established hair analysis technique and has been replicated by independent laboratories across the world (see Magura, Freeman, Siddiqi, & Lipton, 1992). Gas chromatography/mass spectrometry confirmations of RIAH have not revealed any false positives because of testing errors (Magura et al., 1992).
The Timeline Follow-Back Interview (TLFB; Sobell, Sobell, Klajmer, Pavan, & Basian, 1986) was used to assess maternal substance use during pregnancy and postnatally. The TLFB yield data about the average number of joints smoked per week, average number of cigarettes smoked per week, and average number of standard drinks per week during pregnancy. Postnatal substance use was computed by taking the average of number of days used cocaine, number of cigarettes per week, number of standard drinks per week, and number of joints per week from the 18, 24, 30, 36, 42, 48, and 54 month assessments.
Child behavior problems
The externalizing behavior problem subscale of the Child Behavior Checklist (CBCL; Achenbach, 1992) was used as a measure of child externalizing behavior problems. Maternal reports of child behavior problems were obtained during assessments at 18, 24, 30, 36, 42, 48, and 54 months of age using the 1.5 – to 5 year version of the CBCL. Total raw scores for externalizing behavior problems were used in analyses and the unstandardized estimates are presented in the text. Scores ranged from 0 to 46 and higher scores indicate more behavior problems. However, to aid in the clinical interpretation of our findings externalizing behavior problems are scaled using T scores in the figures.
Maternal negative affect
Maternal negative affect was assessed using behavioral observations during a Free Play task at 7 and 13 months. Mothers were asked to interact with their infants as they normally would at home for 10 minutes in a room filled with toys. These interactions were coded using a collection of global 5-point rating scales developed by Clark, Musick, Scott, and Klehr (1980), with higher scores indicating more positive affect or behavior. The scale for maternal negative affect consisted of items such as angry, hostile tone of voice; expressed negative affect; angry, hostile mood; and displeasure or disapproval or criticism. The maternal negative affect scale had high internal consistency with Cronbach’s alpha of .96 at 7 months and .94 at 13 months. Two coders rated maternal behavior. Both coders were trained on the Clark scales by the third author and were unaware of group membership. Inter-rater reliability was conducted on a random selection of 14% (n = 23 and 24 at 7 and 13 months respectively) of the tapes and ranged from an Intraclass correlation coefficient of .82 at 13 months to .96 at 7 months. Because there were no significant differences in maternal affect between the two observation periods, we created a mean maternal negative affect score across the 7 and 13 months observations. Scores ranged from 1.00 to 3.13 and higher scores indicate higher maternal negative affect.
Cumulative caregiving environmental risk
A composite caregiving environmental risk score was computed from the following measures.
Maternal Psychopathology was assessed using the Brief Symptom Inventory (BSI; Derogatis, 1993) at 1, 7, 13, 24, 36 and 48 months. This scale is a brief form of Symptom Checklist 90-R, and is a widely used mental health screening measure in a variety of clinical and research settings. The measure consists of 53 items rated on a five-point scale. The items are grouped into nine scales of Anxiety, Hostility, Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Phobic Anxiety, Paranoid Ideation, and Psychoticism. A total score was computed by taking the sum of all ratings. The BSI subscales have high internal consistency and the measure has been used in a large number of studies, including studies of maternal cocaine use (e.g., Eiden et al., 2007; Singer et al., 2002). Higher scores indicate higher maternal psychopathology.
Maternal Exposure to Violence was assessed at each time point (i.e., 1, 7, 13, 18, 24, 30, 36, 42, 48, and 54 months) using the Timeline Followback Interview (TLFB, Sobell & Sobell, 1996). Although the original interview was a calendar based method to assess substance use, it has also been used to measure episodes of intimate partner violence in various studies (e.g., Mignone, Klostermann, & Chen, 2009). Women were asked about their exposure to violence using a daily calendar at each assessment point. These events included witnessing, experiencing, and/or perpetrating violence. The total number of days women witnessed, experienced, or perpetrated violence were summed within each time point. Given the apparent bimodal distributions at each time point, exposure to violence was dummy-coded (i.e., 0, 1) at each time point and then a count variable was created from each of the dummy-coded time points to reflect exposure to violence across time.
Caregiving Instability was assessed at 1, 7, 13, 24, and 36 months using a structured caregiver interview (Platzman, Coles, Lynch, Bard, & Brown, 2001). The Structured Clinical Interview (SCI) was developed for use with high risk infants from 6 months to 24 months and was administered to the child’s caregiver by a trained examiner. Following Platzman et al. (2001), individual items from the SCI were summed into a cluster called caregiving instability. The five items included in this scale were as follows: no male adult in the household, baby separated from primary caregiver for more than 48 hours, baby is fed and sleeps significantly less than average, there are custody changes, or baby does not see biological mother regularly. Thus, the total score on caregiving instability was based on the presence of absence of the 5 specific risk characteristics described above (see Platzman et al., 2001 for details regarding scale development). The scale is similar to a composite risk index (e.g., Sameroff et al., 1993), based on research suggesting that the total number of risk factors may be more predictive of child outcomes than exposure to any specific risk condition (Seifer, 1995; Zeahah et al., 1997). However, unlike the traditional risk composites or environmental risk scores used in other studies, it is more narrowly focused on factors that are associated with instability in the caregiving environment, particularly among families with drug using mothers. The caregiving instability scores ranged from 0 to 4 in the current study with the majority of the children in the sample having a score of 0 or 1. Thus, caregiver instability was dummy-coded at each time point and a count variable was created from each of the dummy-coded time points reflecting caregiver instability across the time periods. Previous studies have reported significant positive associations between maternal drug use and higher caregiving instability (Eiden, Peterson, & Coleman, 1999; Platzman et al., 2001). The caregiving instability cluster was also associated with an acceleratory heart rate response indicating distress or arousal among cocaine exposed infants (Bard et al., 2000) and was a predictor of child behavior problems among 2 to 5 year old children of cocaine using mothers (Eiden, 1999).
The cumulative caregiving environmental risk variable was created by computing a count variable which included BSI, exposure to violence, and caregiving instability for each time point that each variable was assessed. Specifically, a point was granted when a person had a score in the upper quartile for total BSI for each time point that it was assessed, when a person received a score of 1 for the dummy-coded variable for maternal exposure to violence for each time point that is was assessed, and when a person received a score of 1 for the dummy-coded variable for caregiving instability for each time point that it was assessed. BSI was assessed at six time points (i.e., 1, 7, 13, 24, 36 and 48 months), exposure to violence was assessed at ten time points (i.e., 1, 7, 13, 18, 24, 30, 36, 42, 48, and 54 months), and caregiving instability was assessed at five time points (i.e., 1, 7, 13, 24, and 36 months). Thus, scores for cumulative caregiving environmental risk could theoretically range from 0 to 21. The scores for cumulative caregiving environmental risk ranged from 0 to 17 in this sample.
Statistical Analyses
Multilevel growth curve analyses were conducted using hierarchical linear modeling (HLM version 6.04; Raudenbush, Bryk, & Congdon, 2004). As recommended by Mehta and West (2000), repeated measures of externalizing behavior problems were modeled continuously as a function of age in months (Level 1), and nested within individuals (Level 2). Age in months was centered at baseline (i.e., 18 months of age), and was entered into models uncentered to predict linear and quadratic (i.e., age in months squared) effects of time on externalizing behavior problems. Individual-level variables (i.e., child sex, demographics, PCE, prenatal and postnatal maternal substance use, and maternal negative affect) were modeled at Level 2 and were allowed to predict the intercept, linear, and quadratic age in months coefficients at Level 1 (i.e., cross-level interactions). All terms were entered as fixed effects (intercepts and error terms were allowed to be random). Dichotomous variables (e.g., child sex) were entered uncentered, and all other variables were entered grand-mean centered (Hox, 2010). Interaction terms between two Level 2 variables were created in SPSS, version 19, as a product of the two grand-mean centered variables and entered uncentered in HLM. HLM allows for missing data at Level 1. HLM provides unstandardized estimated effects. Thus, results reported from HLM models in the text are unstandardized coefficients. However, to facilitate translation of reported effects as they pertain to clinical cutoff scores for externalizing behavior problems, any results that are plotted in figures are done so using clinical T scores.
An iterative procedure was used in the estimation of all models. First, to address hypothesis 1, a means only model predicting externalizing behavior problems was tested to allow for the calculation of an intraclass correlation coefficient (ICC). Second, to address hypothesis 2, an unconditional model including the intercept, linear, and quadratic effects of age in months was estimated predicting externalizing behavior problems. Third, to test hypothesis 3, demographic, prenatal, and postnatal maternal substance use involvement variables were then added to the unconditional model to identify significant covariates for inclusion in all remaining models. Potential covariates were chosen based on theory and empirical studies (e.g., Anderson et al., 2003; Bada et al., 2007; Minnes et al., 2010; Chaplin et al., 2009), were grouped conceptually, and tested in blocks. First, demographic variables (i.e., maternal age, education, parity, race, foster care status, and SES) were tested as potential covariates, next perinatal variables were tested as potential covariates (i.e., gestational age, birth weight, and head circumference), prenatal substance exposure (cigarettes, alcohol, and marijuana) was tested next, followed by postnatal substance use (alcohol, cigarettes, marijuana, and cocaine). To improve model parsimony and stability (Raudenbush & Bryk, 2002), nonsignificant terms were trimmed from models. However, because of our hypotheses concerning child sex, caregiving environmental risk, and PCE, these variables were retained and statistically accounted for in all subsequent models regardless of their significance levels. Fourth, to test hypothesis 4, multilevel tests of mediation were conducted following procedures outlined by Krull and MacKinnon (2001) by systematically adding in maternal negative affect to the trimmed models used to test hypothesis 3. Finally, to test hypotheses 5 and 6 regarding moderation by caregiving environmental risk and sex, respectively, interaction terms between PCE and caregiving environmental risk and between PCE and sex were simultaneously added to trimmed models used to test hypothesis 4.
Results
Demographics and perinatal risk
Results from MANOVA with the demographic variables as the dependent measures and PCE status yielded a significant multivariate effect of group status, (F[3, 163] = 6.05, p < .01). Results from univariate analyses indicated that control group mothers were younger, had lower parity, and higher education compared to those in the PCE group, although the effect sizes were generally small (see Table 1). A second MANOVA was conducted with infant birth outcomes as the dependent measures. Results indicated a significant effect of PCE group status on infant birth outcomes, (F[5, 161] = 8.11, p < .001). Univariate analyses indicated that PCE infants had lower gestational age, birth weight, birth length, and cocaine using mothers had higher scores on the obstetrical complications scale compared to those in the control group (see Table 1). 86% of the cocaine exposed and 97% of the comparison infants were full term (i.e., ≥ 37 weeks gestational age). When these analyses were repeated after using gestational age as a covariate, the differences in birth weight and length remained significant (p < .01). However, there were no significant associations between any of the perinatal risk variables and toddler externalizing behavior problems at any time point. Group differences were unchanged when accounting for attrition, with the exception of maternal education. Specifically, the two groups were no longer significantly different from one another in maternal education after accounting for attrition. Means and standard deviations for all level 1 variables at each time point are presented in Table 2. The percentage of toddlers in the clinical range for externalizing behavior problems is also presented in Table 2. Finally, Table 3 includes means, standard deviations, and group differences for all level 2 variables.
Table 1.
Group Differences in Demographic Variables and Birth Outcomes
| Exposure Group: | NPCE | PCE | F value | η2 | ||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | |||
| Demographics: | ||||||
| BM age (range 18–42 years) | 28.57 | 5.60 | 30.99 | 6.05 | 7.13** | .04 |
| BM parity (range 0–13) | 3.28 | 1.66 | 4.25 | 2.27 | 9.93** | .06 |
| Years education (range 8 –18) | 12.26 | 1.85 | 11.62 | 1.93 | 4.78* | .03 |
| Maternal Occupation (range 0–9) | 3.40 | 1.89 | 2.69 | 1.15 | 8.95** | .05 |
| Birth outcomes: | ||||||
| Gestational age (weeks) (range 32–41) | 39.33 | 1.27 | 38.68 | 1.83 | 6.91* | .04 |
| Birth weight (gms) (range 1531–5072) | 3320.15 | 494.58 | 2988.85 | 523.81 | 17.58** | .10 |
| Birth length (cm) (range 32–56) | 49.83 | 2.90 | 48.29 | 3.12 | 10.81** | .06 |
| Head circumference (cm) (range 26.3–48.5) | 35.55 | 1.28 | 33.25 | 2.18 | 1.14 | .01 |
| OCS (range 48–160) | 100.53 | 17.15 | 86.38 | 16.18 | 30.07** | .15 |
| Substance use: | ||||||
| Cigarettes/week (range 0–254.12) | 9.81 | 21.91 | 36.26 | 42.04 | 35.62** | .18 |
| Drinks/week (range 0–68.8) | .06 | .15 | 5.06 | 13.27 | 20.59** | .11 |
| Joints/week (0–52.11) | 1.12 | 6.20 | 1.55 | 4.62 | 2.34 | .01 |
| Days cocaine/week (range 0–6.63) | 0 | 0 | .94 | 1.58 | 28.46** | .15 |
Note.
p < .05
p < .01
Note. BM: biological mother; OCS: Obstetrical complications scale score, high scores are more optimal. Maternal occupation was coded using Hollingshead (1957, 1975) scoring system. Substance use refers to prenatal substance use.
Table 2.
Means and standard deviations for externalizing behavior problems and postnatal substance use from 18 to 54 months of age
| 18 months | 24 months | 30 months | 36 months | 42 months | 48 months | 54 months | ||
|---|---|---|---|---|---|---|---|---|
| Externalizing behavior problems | PCE | 12.41 (8.20) 14% |
12.81 (98.53) 15% |
12.99 (8.39) 18.7% |
12.52 (8.40) 14% |
13.42 (9.68) 18.7% |
11.20 (8.90) 14% |
11.79 (9.43) 12% |
| NPCE | 12.38 (8.69) 15.7% |
11.89 (9.16) 12.4% |
14.16 (10.26) 22.5% |
12.53 (9.46) 14.6% |
13.68 (10.99) 20.2% |
10.64 (8.78) 10.1% |
10.06 (8.79) 7.9% |
|
| Postnatal Alcohol Use | PCE | .44 (1.28) | 2.07 (3.57) | .23 (.68) | 2.56 (4.44) | .26 (.69) | 1.89 (2.90) | .56 (1.90) |
| NPCE | .16 (.68) | 2.87 (3.95) | .08 (.17) | 2.91 (3.88) | .16 (.59) | 2.59 (2.90) | .19 (.39) | |
| Postnatal Cigarette Use | PCE | 6.34 (6.35) | 5.24 (6.40) | 6.60 (6.31) | 6.18 (7.31) | 6.45 (6.32) | 5.64 (7.84) | 5.81 (6.41) |
| NPCE | 3.31 (5.02) | 3.06 (6.10) | 3.77 (5.33) | 2.99 (6.08) | 2.91 (4.78) | 2.71 (4.77) | 3.80 (5.26) | |
| Postnatal Marijuana Use | PCE | .73 (4.73) | .84 (1.79) | .75 (2.14) | 1.02 (2.19) | .48 (1.13) | .54 (1.59) | .35 (1.04) |
| NPCE | .28 (1.20) | .57 (1.43) | .53 (1.56) | .81 (1.71) | .44 (1.44) | .46 (1.31) | .39 (1.08) | |
| Postnatal Cocaine Use | PCE | 3.98 (18.79) | 2.99 (9.52) | 4.28 (18.15) | 5.34 (23.15) | 5.00 (22.73) | 4.05 (20.93) | 3.29 (16.02) |
| NPCE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Note. N = 196. Percentages for externalizing behavior problems refer to the percentage of children who were in the clinical range.
Table 3.
Means, standard deviations, and group differences by PCE status for level 2 model variables
| Exposure Group: | NPCE | PCE | t value | p | ||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | |||
| Maternal Negative Affect | 1.28 | .38 | 1.58 | .61 | −4.31 | <.001 |
| Caregiving Environmental Risk | 4.97 | 3.82 | 5.51 | 3.59 | −1.02 | .31 |
Note. N=196
Hypothesis 1: Individual differences or significant variability in the developmental trajectory of externalizing behavior problems from 18 to 54 months would be found
Calculating the intraclass correlation coefficient showed that two-thirds of the variance in externalizing behavior problems in the present data was explained by individual differences (ρ = .655). That is, an estimated 65% of the total variation in externalizing behavior problems was attributable to individual differences between toddlers.
Hypothesis 2: The average trajectory of externalizing behavior problems in toddlers over time would be normative
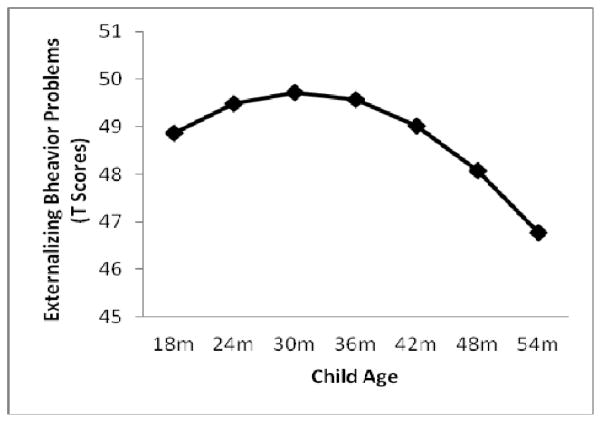
As expected, and as illustrated in Figure 1, the base growth curve model showed that externalizing behavior problems were significantly greater than zero at baseline (b = 12.191, p < .001), significantly increased from 18 to 30 months (b = .117, p = .013), and then significantly decreased from 30 to 54 months (b = −.004, p = .001).
Figure 1.
Average curvilinear trajectory of externalizing behavior problems, plotted in clinical T scores, from 18 to 54 months of age.
Hypothesis 3: PCE would predict trajectories of externalizing behavior problems in toddlers over time
Contrary to expectation, cocaine group status (PCE vs. NPCE) did not predict intercept (b = .662, p = .592), linear (b = −.120, p = .207), or quadratic (b = .004, p = .128) differences in externalizing behavior problem trajectories over time. Analysis with number of days used cocaine during pregnancy (to examine dose response associations) produced similar non-significant results (intercept: b = .302, p = .591; linear: b = −.008, p = .851; quadratic: b = .001, p = .658). These models statistically controlled for the main effects of caregiving environmental risk and child sex (see hypothesis 5 and hypothesis 6 below). Only caregiving environmental risk significantly predicted intercept (but not linear [b = −.004, p = .784] or quadratic [b = .000, p = .754]) differences in externalizing behavior problems (b = .690, p < .001). Additionally, these models tested prenatal alcohol, cigarette, and marijuana exposure; postnatal cocaine, alcohol, cigarette, and marijuana exposure; whether children were in foster care or not; parental socioeconomic status; maternal age; parity; and race as covariates. None of these variables were significant predictors of intercept, linear, or quadratic differences in externalizing behavior problem trajectories and were thus dropped from models to enhance model parsimony.
Hypothesis 4: Maternal negative affect would mediate the association between PCE and trajectories of externalizing behavior problems in toddlers over time
Maternal negative affect was tested as a hypothesized mediator between prenatal cocaine exposure and externalizing behavior problems. Because PCE did not significantly predict externalizing behavior problems (see H3), true mediation cannot occur (Baron & Kenny, 1986). However, indirect effects can still exist between the predictor variable, a third variable, and the outcome variable outside of the presence of direct effects between the predictor and outcome variables (Sobel, 1982; Shrout & Bolger, 2002). As expected, PCE significantly predicted greater maternal negative affect (b = .308, p < .001). Moreover, maternal negative affect significantly predicted externalizing behavior problem trajectories. Maternal negative affect was associated with significantly greater linear increase (b = .233, p = .019) as well as marginally greater quadratic decline (b = −.005, p = .052) in externalizing behavior problems over time, but not with intercept differences (b = −1.305, p = .296). Sobel tests indicated significant indirect linear (2.042, p = .041, two-tailed), but not quadratic (−1.571, p = .116, two-tailed) effects (Sobel, 1982). Thus, in sum, results demonstrated that PCE was indirectly related to a greater linear increase in externalizing behavior problems from about 18 to 30 months via higher levels of maternal negative affect.
Hypothesis 5: Caregiving environmental risk would moderate the effects of PCE on trajectories of externalizing behavior problems in toddlers over time
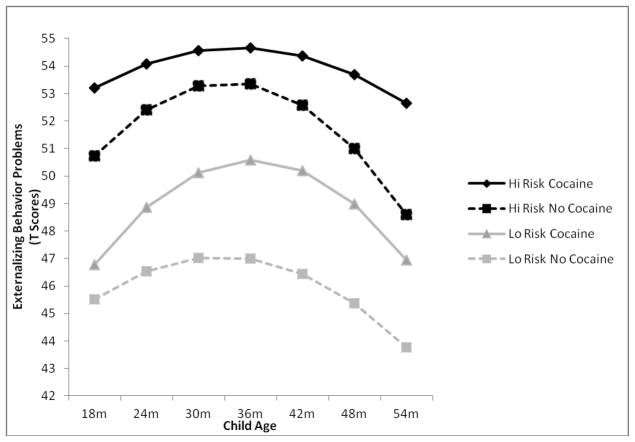
In line with expectation, caregiving environmental risk marginally moderated the linear (b = −.051, p = .051) and significantly moderated the quadratic (b = .001, p = .045) effects of PCE to predict externalizing behavior problem trajectories over time. As shown in Figure 2, differential linear and quadratic growth in externalizing behavior problems was seen depending on levels of PCE and caregiving environmental risk. Specifically, and as expected, PCE children who experienced high caregiving environmental risk had the highest overall trajectory of externalizing behavior problems (intercept: b = 14.510, p < .001; linear: −.023, p = .812; quadratic: b = .000, p = .939), followed by non-PCE children in high risk environments (intercept: b = 13.074, p < .001; linear: b = .339, p = .004; quadratic: b = −.010, p = .001), PCE children in low risk environments (intercept: b = 9.128, p < .001; linear: b = .188, p = .079; quadratic: b = −.006, p = .053), and non-PCE children in low risk environments (intercept: b = 8.948, p < .001; linear: b = .195, p = .048, quadratic: b = −.006, p = .022), respectively. PCE children in high risk environments were the only children to not show quadratic declines in externalizing behavior problems over time. Moreover, these children maintained significantly elevated levels of externalizing behavior problems at 54 months compared to non-PCE children in high risk environments as evidenced by a significant quadratic effect of PCE among high caregiving risk children (b = .010, p = .005). A similar difference in quadratic decline was not found as a function of PCE among children in low risk caregiving environments (b = .000, p = .892). This suggests that the combination of PCE and a high risk caregiving environment is uniquely detrimental to children’s development, as these children have the highest overall levels of externalizing behavior problems during the toddler period and that they do not experience a normative decline in these problems relative to other children with fewer risk factors.
Figure 2.
PCE by caregiving environmental risk interaction predicting differential linear growth and quadratic decline in externalizing behavior problem trajectories, plotted in clinical T scores, from 18 to 54 months of age. Low (Lo Risk) and high (Hi Risk) values of caregiving environmental risk represent the 20th and 80th percentiles of the distribution, respectively.
Hypothesis 6: Does child sex moderate the effects of PCE on trajectories of externalizing behavior problems in toddlers over time?
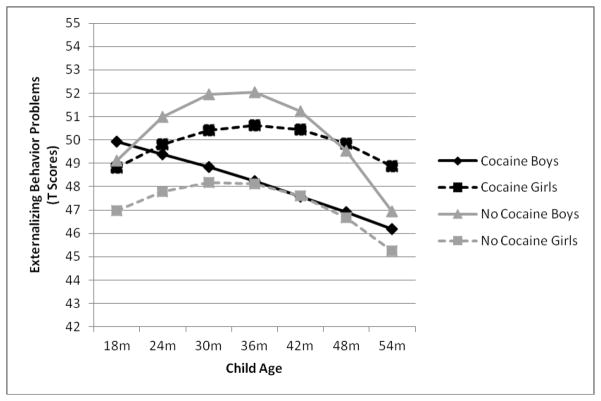
Finally, results indicated that child sex marginally moderated the linear (b = −.369, p = .056), but did not moderate the quadratic (b = .008, p = .113) growth effects of PCE on externalizing behavior problems over time or intercept differences. As shown in Figure 3, the linear interaction was driven by the fact that only PCE boys showed negative linear growth in externalizing behavior problems over time, whereas boys not exposed to cocaine, PCE girls, and girls not exposed to cocaine showed positive growth. However, follow-up tests revealed that this negative linear growth for PCE boys was not statistically significant. The positive linear trends for PCE and non-PCE girls were also not statistically significant. Only boys who were not exposed to cocaine showed a significant positive linear trend (b = .294, p = .004). Thus, despite these linear trends being significantly different from one another, no significant increases in externalizing behavior problems were found as a function of PCE between boys and girls, indicating that child sex did not moderate the association between PCE and externalizing behaviour problem trajectories
Figure 3.
PCE by child sex interaction predicting differential linear growth in externalizing behavior problem trajectories, plotted in clinical T scores, from 18 to 54 months of age.
Discussion
Few studies of PCE have examined indirect pathways to externalizing behavior problems trajectories or moderators of risk other than child sex. Indirect pathways that are prospective may be especially important as they suggest a cascade of events that may predict risk for a particular child outcome, with implications for timing and content of preventive interventions. Results from the current study highlight the role of maternal negative affect during mother-infant play interactions as an important mediator of risk. That is, PCE was indirectly related to a greater linear increase in externalizing behavior problems via higher levels of maternal negative affect. The results are also supportive of diathesis-stress models indicating that a combination of prenatal cocaine exposure and high caregiving environmental risk was associated with the most risky trajectories of externalizing behavior problems in early childhood. Finally, results indicated that child sex did not moderate the association between PCE and externalizing behavior problems.
Overall, toddlers in the present study followed the normative externalizing behavior problem trajectory, with externalizing behavior problems increasing until approximately three years of age and then decreasing from three to four years of age. This pattern reflects the typical development of externalizing behavior problems during this developmental period (e.g., Campbell, 1995; Cummings et al., 1989; Nagin & Tremblay, 1999). Interestingly, PCE did not directly predict trajectories of externalizing behavior problems in the present work. This finding is in line with a number of previous studies that have also reported no direct association between PCE and externalizing behavior problems (e.g., Accornero et al., 2002; Bennett et al., 2002; Chaplin et al., 2009; Richardson et al., 1996). However, it is at odds with studies reporting direct associations between PCE and externalizing behavior problems (e.g., Bada et al., 2007; Richardson et al., 2009). One possible explanation for these mixed findings may be due to differences in amount, duration, and timing of exposure that are difficult to determine. A second explanation is variations in quality of care including custody changes and type of foster care across different studies. A third explanation may be differences in sample size that may lead to non-significant findings for small effects, and control for different covariates. Finally, a fourth explanation may be the role of cumulative caregiving environmental risk and the possibility of indirect pathways in the absence of direct associations. Indeed, the present work importantly demonstrates that the effects of PCE on externalizing trajectories before school age depend on specific mediating and moderating variables. Evidence from studies using neuroimaging indicate subtle differences between PCE and control children in brain structure and function in cortical areas that may increase over time, since these areas continue to develop throughout childhood and adolescence (see Ackerman, Riggins, & Black, 2010). Thus, developmental trajectories of externalizing problems among PCE compared to non-PCE children may be quite different in later childhood and adolescence.
Although there were no direct effects of PCE, results indicated that there was a significant indirect association between PCE and trajectories of externalizing behavior problems via maternal negative affect. These findings are in line with previous studies of substance exposed children. Yumoto et al. (2008), for instance, found that the emotional responsiveness of the primary caregiver and the emotional climate of the home were the most important predictors with regards to behavioral outcomes such as aggression and delinquency in school. Moreover, Bailey et al. (2012) found that the link between early illicit drug use disorder among parents and children’s externalizing behavior problems was accounted for by parental negative affect. More specific to PCE, Bennett et al. (2002) reported that other facets of parenting, such as harsh discipline, predicted behavior problems in PCE children. Finally, Eiden et al. (2011) found a similar pattern of results when examining the association between PCE and self-regulation at three years of age, as they found that maternal harshness mediated the link between PCE and self-regulation. These results are further supported by evidence from animal models indicating that gestational cocaine treatment results in overtly aggressive behavior towards an intruder (Heyser, Molina, & Spear, 1992; Johns et al., 1994; Johns et al., 1998). Moreover, subsequent disruption in maternal care and increased aggression can be “transmitted” to next generation offspring either through prenatal exposure to cocaine or through the rearing experience when reared by a cocaine treated dam (Johns et al., 2005; McMurray et al., 2008). Thus, maternal parenting behavior may be a critical pathway to greater linear increases in externalizing behavior problems in the toddler period among cocaine exposed toddlers.
The results of the present work also highlight the nested quality of risk characteristics as described by transactional models of development (Rutter, 1987; Sameroff et al., 1993). While the characteristics under study may independently predict the development of externalizing behavior problems, transactional theorists underscore the importance of examining aggregations of risk, granting an opportunity to study the general context of development concerning parent, child, and family characteristics. This approach is especially appropriate when studying PCE children who often vary considerably in risk structure (Brown et al., 2004; Eiden et al., 2007).
With a few exceptions (see Bennett et al., 2002), the majority of studies of PCE have investigated the effects of prenatal exposure after statistically accounting for the effects of environmental risk. However, our results are supportive of a vulnerability model (Luthar & Zelazo, 2003) indicating that in the presence of prenatal cocaine exposure, higher caregiving environmental risk confers vulnerability by predisposing children to the highest average levels of externalizing behavior problems and by slowing the normative declines in these problems in the preschool years. Additionally, these findings statistically accounted for the mediating effects of maternal negative affect, suggesting that the detrimental impact of caregiving environmental risk extends beyond the impact of maternal negative affect. The present findings are also in line with animal studies that have revealed links between PCE and increased sensitivity to environmental stressors (Sobrian et al., 1990; Spear et al., 1998).
Finally, although initial results indicated an interaction between PCE and child sex in the prediction of externalizing behavior trajectories, follow-up tests revealed that the association between PCE and these trajectories did not vary for boys and girls. Thus, the present work failed to provide clear evidence that child sex moderates the association between PCE and trajectories of externalizing behavior problems. The current findings are in line with previous studies of PCE that have examined sex differences in externalizing behavior problems. Results across these studies are generally mixed, with some studies indicating that the association varies by child sex, although the direction differs across studies (Bailey et al., 2005; Delaney-Black et al., 2004; Sood et al., 2005), while others indicate no sex-related differences or moderation by sex (Bada et al., 2011). However, many of the studies reporting sex-related differences consist of older children. It is possible that the demand of the school context at higher grades leads to higher variability in child behavior problems. Thus, the association between cocaine exposure and externalizing behavior problems may begin to vary as a function of child sex at older ages. Another possibility is that there are sex-related differences in the extent to which children “mature out” of externalizing problems in general (Colder, Mott, & Berman, 2002) and in substance exposed samples of children (Edwards et al., 2006), resulting in more apparent sex-related differences at older ages. Future studies examining prospective changes in externalizing behavior problems from preschool to the school years may be better able to address this question.
Limitations
First, the measure of externalizing behavior problems was only assessed via maternal report. The depression-distortion hypothesis (Richters & Pelligrini, 1989) indicates that dysphoria associated with maternal depression and other psychopathology may activate a negative perceptual bias in maternal ratings of child behavior, leading to the over-reporting of child behavior problems. However, it is difficult to attain other evaluations of child behavior problems at these ages, especially for samples with low levels of male caregiver involvement.
Second, although care was taken in this study to identify substance use in this sample, the accurate assessment of substance use is problematic. Pregnant and postpartum women are often reluctant to disclose information concerning their substance use, especially of illicit substances such as cocaine. A strength of the present work; however, was the use of multiple methods to assess prenatal substance use, which to a degree mitigated this limitation even though the urine toxicology information was abstracted from medical records. There were, nonetheless, no biological markers employed in the present work for assessing prenatal alcohol consumption or cigarette smoking. It is conceivable that mothers may have misrepresented their use of these substances as a result of inaccurate memory or refusal to disclose usage.
In conclusion, the results of this study built upon previous literature examining the role of PCE on trajectories of externalizing behavior problems. The present work both clarified and highlighted the important role of mediators and moderators of the link between PCE and externalizing behavior problems. More specifically, the findings of this study underscored the importance of maternal negative affect as a mediator of the relationship between PCE and trajectories of externalizing behavior problems. Results also highlight the role of cumulative caregiving environmental risk, and indicate that cocaine exposed children experiencing lower vs. higher caregiving environmental risk may begin to follow different developmental trajectories for externalizing problems in the early preschool years. Overall, the results are supportive of diathesis-stress and vulnerability models indicating that the combination of PCE and cumulative caregiving risk is associated with the highest risk trajectories for externalizing behavior problems.
Acknowledgments
The authors thank the families who participated in this study and the research staff responsible for data collection. Special thanks to Dr. Claire Coles for collaboration on the larger study, Dr. Amol Lele for collaboration on data collection at Women and Children’s Hospital of Buffalo, and Dr. Michael Ray for his collaboration on data collection at Sisters of Charity Hospital of Buffalo. The work described was supported by Award Number R01DA013190 from the National Institute On Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Accornero VH, Morrow CE, Xue L, Bandstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed prenatally to cocaine: Role of maternal behavioural health. Journal of Pediatric Psychology. 2002;27:259–269. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. Manual for the Child Behavior Checklist/2–3 and 1992 profile. 1992. Unpublished manuscript. [Google Scholar]
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554– 565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Publication manual of the American Psychological Association. 5. Washington, D.C: 2001. [Google Scholar]
- Anderson P, Doyle LW the Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. Journal of the American Medical Association. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Bada HS, Bann CM, Bauer CR, Shankaran S, Lester B, LaGasse L, Higgins R. Preadolescent behavior problems after prenatal cocaine exposure: Relationship between teacher and caretaker ratings (Maternal Lifestyle Study) Neurotoxicology and Teratology. 2011;33:78–87. doi: 10.1016/j.ntt.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, et al. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicology and Teratology. 2005;27:181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Hill KG, Guttmannova K, Oesterle S, Hawkins JD, Catalano RF, McMahon RJ. The Association Between Parent Early Adult Drug Use Disorder and Later Observed Parenting Practices and Child Behavior Problems: Testing Alternate Models. Developmental Psychology. 2012 Jul 16; doi: 10.1037/a0029235. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Developmental Psychobiology. 2000;36:194–212. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Social and Personality Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauman PS, Dougherty FE. Drug-addicted mothers’ parenting and their children’s development. International Journal of the Addictions. 1983;18:291–302. doi: 10.3109/10826088309039348. [DOI] [PubMed] [Google Scholar]
- Beaudin SA, Gendle MH, Strupp BJ. Gender influences on the cognitive and emotional effects of prenatal cocaine exposure: Insights from an animal model. In: Lewis M, Kestler L, editors. Gender differences in prenatal substance exposure. Washington, DC: American Psychological Association; 2011. pp. 77–96. [Google Scholar]
- Bendersky M, Alessandri S, Gilbert P, Lewis M. Characteristics of pregnant substance abusers in two cities in the northeast. American Journal of Drug and Alcohol Abuse. 1996;22:349–362. doi: 10.3109/00952999609001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Developmental Psychology. 2002;38:648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a fuction of prenatal cocaine exposure and gender. Journal of Developmental & Behavioral Pediatrics. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Externalizing problems in fifth grade:Relations with productive activity, maternal sensitivity, and harsh parenting from infancy through middle childhood. Developmental Psychology. 2007;43:1390–1401. doi: 10.1037/0012-1649.43.6.1390. [DOI] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Claire DC, Platzman KA, Lynch ME. Prenatal cocaine exposure: A comparison of 2-year-old children in parental and nonparental care. Child Development. 2004;75:1282–1295. doi: 10.1111/j.1467-8624.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- Burns K, Chethik L, Burns W, Clark R. The early relationship of drug abusing mothers and their infants: an assessment at eight to twelve months of age. Journal of Clinical Psychology. 1997;53:279–287. doi: 10.1002/(sici)1097-4679(199704)53:3<279::aid-jclp11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Campbell SB. Behavior problems in preschool children: A review of recent research. Journal of Child Psychology and Psychiatry. 1995;36:113–149. doi: 10.1111/j.1469-7610.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Pierce EW, Moore G, Marakovitz S, Newby K. Boys’ externalizing problems at elementary school age: Pathways from early behavior problems, maternal control, and family stress. Development and Psychopathology. 1996;8:701–719. [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicology and Teratology. 2009;31:275–282. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Musick J, Scott F, Klehr K. The Mothers’ Project Rating Scale of Mother–Child Interaction. 1980. Unpublished manuscript. [Google Scholar]
- Colder CR, Mott JA, Berman AS. The interactive effects of infant activity level and fear on growth trajectories of early childhood behavior problems. Development and Psychopathology. 2002;14:1–23. doi: 10.1017/s0954579402001013. [DOI] [PubMed] [Google Scholar]
- Conner-Burrow NA, Johnson B, Whiteside-Mansell L. Maternal substance abuse and children’s exposure to violence. Journal of Pediatric Nursing. 2009;24:360–368. doi: 10.1016/j.pedn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Iannotti RJ, Zahn-Waxler C. Aggression between peers in early childhood: Individual continuity and developmental change. Child Development. 1989;60:887–895. doi: 10.1111/j.1467-8624.1989.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory (BSI) administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, et al. Prenatal cocaine: quantity of exposure and gender moderation. Development Behavior Pediatric. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Kurtz PF, Chin MD. A systematic review of challenging behaviors in children exposed prenatally to substances of abuse. Research in Developmental Disabilities. 2008;29:483–502. doi: 10.1016/j.ridd.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Edwards E, Eiden R, Colder CR, Leonard KE. The development of aggression in 18 to 48 month old children of alcoholic parents. Journal of Abnormal Child Psychology. 2006;34(3):393–407. doi: 10.1007/s10802-006-9021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD. Exposure to violence and behavior problems during early childhood. Journal of Interpersonal Violence. 1999;14:1299–1313. [Google Scholar]
- Eiden RD, Foote A, Schuetze P. Maternal cocaine use and caregiving status: Group differences in caregiver and infant risk variables. Addictive Behaviors. 2007;32:465–476. doi: 10.1016/j.addbeh.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Granger DA, Schuetze P, Veira Y. Child behavior problems among cocaine-exposed toddlers: Indirect and interactive effects. Development and Psychopathology. 2011;23:539–550. doi: 10.1017/S0954579411000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Peterson M, Coleman T. Maternal cocaine use and the caregiving environment during early childhood. Psychology of Addictive Behaviors. 1999;13:293–302. [Google Scholar]
- Eiden RD, Schuetze P, Veira Y, Cox E, Jarrett T, Johns J. Cocaine exposure and children’s self-regulation: Indirect association via maternal aggression. Frontiers in Psychiatry. 2011;2:31. doi: 10.3389/fpsyt.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Schuetze P, Colder CR, Veira Y. Maternal cocaine use and mother-toddler aggression. Neurotoxicology and Teratology. 2011;33:360–369. doi: 10.1016/j.ntt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M, Hinnant JB, Cummings EM. Skin conductance level reactivity moderates the longitudinal association between harsh parenting and child externalizing behavior. Developmental Psychology. 2011;47:693–706. doi: 10.1037/a0021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Dishion TJ, Kaufman NK. Depressed mood and maternal report of child behavior problems: Another look at the depression–distortion hypothesis. Journal of Applied Developmental Psychology. 2009;30:149–160. doi: 10.1016/j.appdev.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH. Cocaine and other stimulants: Action, abuse, and treatment. New England Journal of Medicine. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Gottwald SR, Thurman SK. The effects of prenatal cocaine exposure on on mother-infant interaction and infant arousal in the newborn period. Topics in Early Childhood Special Education. 1994;14:217–231. [Google Scholar]
- Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicology & Teratology. 1992;14:415–421. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Multilevel analysis: Techniques and applications. 2. New York: Routledge; 2010. [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, Haslup AM, Middleton CL, Elliott JC, Walker CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behavioral Neuroscience. 2005;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behavioral Neuroscience. 1994;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, McMillen BA, Means LW, Walker CH, et al. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and in their prenatally exposed offspring. Ann NY Acad Sci. 1998;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- Johnson AL, Morrow C, Accornero VH, Xue L, Anthony JC, Bandstra ES. Maternal cocaine use: Estimated effects on mother-child play interactions in the preschool period. Journal of Developmental Behavioral Pediatrics. 2002;23:191–202. doi: 10.1097/00004703-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behavioral Research. 2001;36:249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Messinger D, Lester BM, et al. Prenatal drug exposure and maternal and infant feeding behavior. Archives of Disease in Childhood: Fetal and Neonatal. 2003;88:F391–F399. doi: 10.1136/fn.88.5.F391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Increased ‘default mode’ activity in adolescents prenatally exposed to cocaine. Human Brain Mapping. 2011;32(5):759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman A, Parmelee B. Medical correlation of infant development. Pediatrics. 1978;61:470–474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Liu J, Lester BM. Reconceptualizing in a dual-system model the effects of prenatal cocaine exposure on adolescent development: a short review. [Review] International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2011;29:803–809. doi: 10.1016/j.ijdevneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Luthar S, Zelazo L. Research on resilience: An integrative review. In: Luthar S, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. New York: Cambridge University Press; 2003. pp. 510–549. [Google Scholar]
- Magura S, Freeman RC, Siddiqi Q, Lipton DS. The validity of hair analysis for detecting cocaine and heroin use among addicts. International Journal of the Addictions. 1992;27:51–69. doi: 10.3109/10826089109063462. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioural teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology & Teratology. 2002;3:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, Jarrett TM, Elliott DL, Black MA, Hofler VE, Walker CH, Johns JM. Intergenerational effects of cocaine treatment and rearing environment on aggressive behavior and oxytocin in rats. Stress. 2008;11:398–410. doi: 10.1080/10253890701850239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, West SG. Putting the individual back into individual growth curves. Psychological Methods. 2000;5:23–43. doi: 10.1037/1082-989x.5.1.23. [DOI] [PubMed] [Google Scholar]
- Mignone T, Klostermann K, Chen R. The relationship between relapse to alcohol and relapse to violence. Journal of Family Violence. 2009;24:497–505. [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, Queh D. The effects of prenatal cocaine exposure on problem behavior in children 4 – 10 years. Neurotoxicology and Teratology. 2010;32:443–451. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JL, Clarke-Stewart A. Trajectories of externalizing behavior from age 2 to age 9: Relations with gender, temperament, ethnicity, parenting, and rater. Developmental Psychology. 2008;44:771–786. doi: 10.1037/0012-1649.44.3.771. [DOI] [PubMed] [Google Scholar]
- Molitor A, Mayes LC, Ward A. Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Development and Psychopathology. 2003;15:39–54. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semi-parametric, group-based approach. Psychological Methods. 1999;4:139–177. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Nagin DS, Tremblay R. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and non-violent juvenile delinquency. Child Development. 1999;70:1181–1196. doi: 10.1111/1467-8624.00086. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Evrard P, Courtoy PJ. Selective direct toxicity of cocaine on fetal mouse neurons. In: Harvey JA, Kosofsky BE, editors. Cocaine: Effects on the developing brain. New York: New York Academy of Sciences; 1998. pp. 51–68. [PubMed] [Google Scholar]
- Nelson CJ, Meter KE, Walker CH, Ayers AA, Johns JM. A dose-response study of chronic cocaine on maternal behavior in rats. Neurotoxicology and Teratology. 1998;20:657–660. doi: 10.1016/s0892-0362(98)00016-6. [DOI] [PubMed] [Google Scholar]
- Olson SL, Lopez-Duran N, Lunkenheimer ES, Chang H, Sameroff AJ. Individual differences in the development of early peer aggression: Integrating contributions of self-regulation, theory of mind, and parenting. Development and Psychopathology. 2011;23:253–266. doi: 10.1017/S0954579410000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzman KA, Coles CD, Lynch ME, Bard KA, Brown JV. Assessment of the caregiving environment and infant functioning in polydrug families: Use of a structured clinical interview. Infant Mental Health Journal. 2001;22:351–373. [Google Scholar]
- Pungello EP, Kupersmidt JB, Burchinal MR, Patterson C. Environmental risk factors and children’s achievement from middle childhood to early adolescence. Developmental Psychology. 1996;32:755–767. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 hierarchical and nonlinear modeling. Chicago, IL: Scientific Software International; 2004. [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: Effects on the development of school-age children. Neurotoxicology and Teratology. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Wilford J. Continued effects of prenatal cocaine use: Preschool development. Neurotoxicology and Teratology. 2009;31:325–333. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters J, Pelligrini D. Depressed mothers’ judgments about their children: An examination of the depression–distortion hypothesis. Child Development. 1989;60:1068–1075. doi: 10.1111/j.1467-8624.1989.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Hastings P, Chen X, Stewarts S, McNichol K. Intrapersonal and maternal correlates of aggression, conflict, and externalizing problems in toddlers. Child Development. 1998;69:1614–1629. [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Dwyer KM, Hastings PD. Predicting preschoolers’ externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology. 2003;39:164–176. [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. American Journal of Orthopsychiatry. 1987;147:598–611. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Garmezy N. Developmental psychopathology. In: Mussen PH, editor. Handbook of child psychology. Vol. 4. New York: Wiley; 1983. pp. 775–912. [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child Development. 1993;64:80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Seifer R. Perils and pitfalls of high-risk research. Developmental Psychology. 1995;31:420–424. [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, et al. Cognitive outcomes of preschool children with prenatal cocaine exposure. Journal of the American Medical Association. 2004;291:2448–3456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicology and Teratology. 2002;24:127–135. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methodology. 1982;13:290–312. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- Sobell MB, Sobell LC, Klajmer F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history. Addictive Behaviors. 1986;11:149–162. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Burton LE, Robinson NL, Ashe WK, James H, Stokes DL, Turner LM. Neurobehavioral and immunological effects of prenatal cocaine exposure in rats. Pharmacology Biochemistry and Behavior. 1990;35:617–629. doi: 10.1016/0091-3057(90)90299-w. [DOI] [PubMed] [Google Scholar]
- Sood BG, Nordstrom, Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, Hannigan JH, Delaney-Black V. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicology & Teratology. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Spear LP, Campbell J, Snyder K, Silveri MM, Katovic NM. Animal behavior models: Increased sensitivity to stressors and other environmental experiences after prenatal cocaine exposure. In: Harvey JA, Kosofsky BE, editors. Cocaine: Effects on the developing brain. New York: New York Academy of Sciences; 1998. pp. 76–88. [PubMed] [Google Scholar]
- Stormshak EA, Bierman KL, McMahon RJ, Lengua L Conduct Problems Prevention Research Group. Parenting practices and child disruptive behavior problems in early elementary school. Journal of Clinical Child Psychology. 2000;29:17–29. doi: 10.1207/S15374424jccp2901_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behavioral Neuroscience. 1996;110:315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high risk youth. Development and Psychopathology. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Hou W, Garvan CW, Wobie K, Eyler FD. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. Journal of Developmental & Behavioral Pediatrics. 2006;27:83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NS, Eyler FD, Behnke M, Conlon M. Cocaine use during pregnancy: Maternal depressive symptoms and infant neurobehavior over the first month. Infant Behavior & Development. 1993;16:83–98. [Google Scholar]
- Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child Development. 2008;79(6):1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Boris NW, Larrieu JA. Infant development and developmental risk: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:165–178. doi: 10.1097/00004583-199702000-00007. [DOI] [PubMed] [Google Scholar]