Abstract
BACKGROUND
Neoadjuvant chemoradiotherapy followed by tumor resection and postoperative chemotherapy is the standard of care for patients with clinical stage II or III adenocarcinoma of the rectum. Significant variation exists in the receipt of postoperative chemotherapy after resection in this population. We seek to understand demographic and clinicopathologic factors associated with initiation of postoperative chemotherapy in elderly patients with rectal cancer to identify potential targets for reducing treatment variation.
METHODS
A retrospective cohort study was performed of rectal cancer patients aged 66 to 80 years old treated by neoadjuvant chemoradiotherapy and radical resection in the Surveillance, Epidemiology, and End Results-linked Medicare database (1998–2007). Multivariate logistic regression was used to assess chemotherapy utilization in relation to patient, tumor and treatment response characteristics.
RESULTS
Among 1492 patients who met study criteria, 61.5% received adjuvant therapy with 5-fluorouracil. Pathologic stage was the strongest determinant of post-operative chemotherapy (48.3% in stage I, 59.6% in stage II, 77.6% in stage III). Increasing age and postoperative readmission were also significantly associated with a decreased rate of adjuvant therapy initiation.
CONCLUSIONS
Although standard treatment guidelines for locally advanced rectal cancer includes postoperative chemotherapy for all patients after neoadjuvant chemoradiotherapy and radical resection, over 1 in 3 patients failed to receive adjuvant therapy. Despite the absence of established evidence, treatment decisions appear to be influenced by the findings at surgical pathology.
Keywords: Rectal cancer, Neoadjuvant therapy, Adjuvant therapy, Chemotherapy, SEER, SEER-Medicare
Rectal cancer is estimated to affect 42,820 individuals in 2013 and account for approximately a third of the 50,830 colorectal cancer deaths.1 Adherence to evidence-based colorectal cancer treatment guidelines is variable and previous studies have shown that patients frequently do not receive adjuvant therapy for cancer of the colon and rectum.2 Reasons for omission of adjuvant therapy are not always apparent, but may include nonclinical factors such as patient preference, provider beliefs, and access to care, in addition to clinical factors such as patient comorbidity, surgical complications, and prolonged post-operative recovery.
The current standard of care for stage II and III rectal adenocarcinoma is neoadjuvant chemoradiotherapy followed by radical resection and adjuvant chemotherapy. Multiple clinical trials have demonstrated a benefit to local control using this approach with a potential for survival benefit as well.3, 4 While this course of therapy often results in substantial tumor regression, current recommendations are based on clinical staging, not post-therapy pathologic staging. However, it is increasingly becoming recognized that response to neoadjuvant chemoradiotherapy may signal tumor chemosensitivity and is an important biomarker for long term outcome.5, 6 Clinical guidelines, such as those from the National Comprehensive Cancer Network (NCCN), recommend that all patients who receive neoadjuvant chemoradiation receive adjuvant chemotherapy after resection, yet it is clear that not all patients do so, even in specialty cancer centers.7, 8 What factors contribute to the omission of chemotherapy after tumor resection is not well understood but variability in treatment and outcomes has been linked to socioeconomic status, race, age, and other factors.9–13 However, in patients who receive neoadjuvant therapy, poor access to care is unlikely to be a major contributor to non-receipt of adjuvant chemotherapy, as they have established relationships with a medical oncologist. We sought to understand what factors are associated with receipt of adjuvant therapy in rectal cancer patients following neoadjuvant chemoradiation and radical resection. Additionally, we examined the temporal trends in administration of adjuvant therapy, including the use of multi-agent regimens.
PATIENTS AND METHODS
This study used the linked Surveillance, Epidemiology, and End Results (SEER) -Medicare database 2009 release, including all Medicare eligible subjects appearing in the SEER dataset from 1991 to 2007 and their Medicare claims through December 2009. The National Cancer Institute (NCI) SEER program is a comprehensive source of population-based information on cancer incidence and patient survival data from designated cancer registries in the United States, representing approximately 26% of the US population.14
Medicare provides health insurance to 97% of individuals aged ≥65 years in the United States and is comprised of Part A covering inpatient and home health care and Part B which provides more comprehensive benefits including outpatient care and physician services.15 The Medicare database complements SEER with treatment and diagnosis details and dates of service.
Approximately 93 percent of patients age 65 and older in the SEER files were successfully linked to the Medicare enrollment file.16 The linked SEER-Medicare data are contained in a series of files including Patient Entitlement and Diagnosis Summary File (PEDSF) (socioeconomic status and Medicare and health maintenance organization (HMO) enrollment information), Medicare Provider Analysis and Review (MEDPAR) file (Part A claims), National Claims History (NCH) file (Part B claims) and Outpatient Standard Analytic File (SAF) (Part B claims).
Patients 66 to 80 years old with pathologic stage I–III rectal (SEER site recode 26) adenocarcinoma (SEER histology codes 8140, 8210–11, 8220–21, 8260–63, 8480–81, 8490) in 1998–2007 were selected for inclusion. Stage was based upon the American Joint Committee on Cancer (AJCC) 7th edition. Patients with stage I disease following neoadjuvant treatment were included as these patients are expected to have had clinical stage II–III cancers prior to neoadjuvant treatment. Patients aged 65 were excluded because lack of claims preceding diagnosis precluded comorbidity index estimation. Since chemotherapy and radiation were principally delivered in non-institutional settings, continuous enrollment in both Medicare parts A and B was required from 12 months preceding diagnosis (to allow measurement of prior comorbidity) through the earliest of 24 months after diagnosis, death, or December 31, 2009. Medicare beneficiaries who participated in HMOs were excluded to ensure completeness of claims. Other exclusion criteria included month of diagnosis undocumented, second primary cancer diagnosis within 24 months, or diagnosis initially noted on nursing/convalescent home/hospice summary, death certificate or autopsy report.
Patients with primary tumor resection (PTR) performed within 6 months after diagnosis were identified. We searched MEDPAR, NCH, and SAF files to identify the earliest claim indicating PTR.17 Local excision or ostomy were not considered definitive surgery. We used the first day of the month of diagnosis to estimate the interval to the date of service. We then identified patients who received both neoadjuvant pelvic radiation and 5-fluorouracil (5-FU) chemotherapy before PTR; patients with claims consistent with radiotherapy18 and chemotherapy19 in MEDPAR, NCH, or SAF files were considered neoadjuvant chemoradiation recipients.
The primary outcome of interest in this study was the initiation of adjuvant chemotherapy among stage I-III rectal cancer patients who received neoadjuvant chemoradiation. Following chemoradiation and PTR, patients with claims indicating 5-fluorouracil-based chemotherapy utilization in MEDPAR, NCH, or SAF files at any point within the 6-month postoperative period were considered as having had adjuvant chemotherapy. After 2004 claims indicating the use of oxaliplatin could be identified.
Patient age at diagnosis, gender, race, and marital status, socioeconomic status, diagnosis year, tumor stage, tumor grade, SEER-based geographical location, and residence were obtained from PEDSF. Socioeconomic status (SES) was determined based on median annual household income, percentage of persons ages 25+ with < 12 years education and percentage of persons living below poverty line based on Census 1990 and 2000 data. Because these variables were highly correlated with each other, they were standardized and equally weighted to create a composite SES variable for analysis, which was then categorized into quartiles.
We calculated comorbidity score according to methodology by Charlson and Romano.20, 21 We searched both MEDPAR and NCH files for 19 pre-defined non-cancer illnesses coded 1 year before to 1 month after the date of diagnosis to create comorbidity scores that were categorized into 0, 1, or ≥2 comorbid conditions according to the NCI-provided SAS macro (available at URL: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html). Postoperative readmissions were defined as occurring within 30 days of operation and identified from the MEDPAR file.
Socioeconomic and clinical variables for all patients and their relationship to adjuvant chemotherapy initiation were assessed using chi-square tests. Multivariate logistic regression analysis was used to assess the relation of these variables to initiation of adjuvant chemotherapy, while controlling for potential confounding effects of patient demographics, tumor factors, and treatment-related variables. Odds ratios (OR) and 95% confidence intervals (CI) were derived. All reported P-values are two-sided and considered significant at the 0.05 level. We used SAS (version 9.1.3; SAS Institute, Cary, NC) for data processing and Stata MP (version 11.0; StataCorp, College Station, TX) for statistical analyses.
RESULTS
We identified 1,492 patients who received chemoradiation prior to rectal resection from the SEER-Medicare linked database and met our inclusion criteria in the years 1998–2007. (Table 1) Patient characteristics are shown in Table 2. Over one third were between the ages of 66–70 (n=567, 38.0%), with a similar number between 71 and 75 (n=555, 37.2%). The majority of patients were male (n=879, 58.9%), White (n=1,340, 89.8%), and resided in large metropolitan or metropolitan areas (n=782, 52.4% and n=423, 28.4% respectively). A slightly larger proportion of patients underwent low anterior resection (LAR) (n=658, 44.1%) vs. abdominoperineal resection (APR) (n=596, 39.9%).
Table 1.
Patient selection and exclusion criteria.
| Criteria | N | Percent of initially identified cases remaining in the analytic cohort |
|---|---|---|
| Stage I–III rectal adenocarcinoma patients treated with chemoradiation 1998–2007 (n=1982) | 1982 | 100 |
| Excluding patients with 2nd primary cancer diagnosis within 24 months (n=133) | 1849 | 93 |
| Excluding survival of less than 6 months after primary tumor resection (n=114) | 1735 | 88 |
| Excluding non-confirmed diagnosis or diagnosis noted on nursing/convalescent home/hospice, death certificate or autopsy report (n=4) | 1731 | 87 |
| Excluding age >80 (n=229) | 1502 | 76 |
| Excluding missing SES (n=10) | 1492 | 75 |
Table 2.
Baseline characteristics of neoadjuvant chemoradiation-treated stage I–III rectal cancer patients and the proportion with adjuvant chemotherapy initiated (N=1,492)
| Characteristics | Patients | Proportion initiating adjuvant chemotherapy within 6-month after surgery, % | |
|---|---|---|---|
| N | Column % | ||
| Overall | 1,492 | 100 | 61.5 |
| Age at diagnosis, yrs | P=0.001 | ||
| 66–70 | 567 | 38 | 67.4 |
| 71–75 | 555 | 37.2 | 58.9 |
| 76–80 | 370 | 24.8 | 56.5 |
| Gender | P=0.652 | ||
| Male | 879 | 58.9 | 62.0 |
| Female | 613 | 41.1 | 60.8 |
| Race/ethnicity | P=0.461 | ||
| White | 1,340 | 89.8 | 61.1 |
| Black | 65 | 4.4 | 61.5 |
| Other/unknown | 87 | 5.8 | 67.8 |
| Composite social-economic status, quartile | P=0.039 | ||
| Top | 373 | 25 | 67.8 |
| 2nd | 373 | 25 | 59.5 |
| 3rd | 373 | 25 | 59.8 |
| Bottom | 373 | 25 | 59 |
| Residence | P=0.246 | ||
| Big Metro | 782 | 52.4 | 64.1 |
| Metro | 423 | 28.4 | 59.1 |
| Urban | 99 | 6.6 | 55.6 |
| Less Urban | 136 | 9.1 | 58.1 |
| Rural | 52 | 3.5 | 63.5 |
| Post-therapy pathologic stage | P<0.001 | ||
| I | 294 | 19.7 | 48.3 |
| II | 502 | 33.6 | 59.6 |
| III | 366 | 24.5 | 77.6 |
| Unknown | 330 | 22.1 | 58.5 |
| Grade | P=0.160 | ||
| Well and moderately differentiated | 1,069 | 71.6 | 61.5 |
| Poorly differentiated and undifferentiated | 226 | 15.1 | 65.9 |
| Unknown | 197 | 13.2 | 56.9 |
| Comorbidity index | P=0.169 | ||
| 0 | 1,037 | 69.5 | 63 |
| 1 | 341 | 22.9 | 59.2 |
| 2+ | 114 | 7.6 | 55.3 |
| Postoperative readmission | P<0.001 | ||
| No | 1310 | 87.8 | 63.9 |
| Yes | 182 | 12.2 | 43.9 |
| Year of diagnosis | P=0.044 | ||
| 1998–1999 | 142 | 9.5 | 57.7 |
| 2000–2001 | 296 | 19.8 | 56.4 |
| 2002–2003 | 343 | 23 | 59.8 |
| 2004–2005 | 361 | 24.2 | 63.4 |
| 2006–2007 | 350 | 23.5 | 67.1 |
| Type of surgery | P=0.010 | ||
| APR | 596 | 39.9 | 58.9 |
| LAR | 658 | 44.1 | 63.5 |
| Other surgery* | 238 | 16.0 | 51.1 |
| SEER region | P=0.075 | ||
| West | 589 | 39.5 | 59.4 |
| Midwest | 225 | 15.1 | 60.4 |
| Northeast | 345 | 23.1 | 67.5 |
| South | 333 | 22.3 | 59.8 |
Other unspecified resection of large intestine
Overall, 61.5% of patients who underwent rectal resection following neoadjuvant chemoradiation subsequently received adjuvant chemotherapy. On univariate analysis, older patients were less likely to initiate adjuvant therapy; while 67.4% of patients aged 66–70 initiated adjuvant treatment, only 56.5% of those 76–80 did so (p=0.001). Patients with higher socioeconomic status initiated adjuvant chemotherapy more often than those from lower strata (67.8% in highest quartile vs. 59.0% in lowest, p=0.039), as did those diagnosed more recently (67.1% of those diagnosed 2006–2007 vs. 57.7% of those diagnosed 1998–1999, p=0.044). Readmission within 30 days of operation occurred for 12.2% of patients. Readmisssion was associated with a decreased rate of initiation of adjuvant chemotherapy on univariate analysis (43.9% vs. 63.9% for those without readmission, p<0.001) Patients with higher post-treatment pathologic stage tumors were more likely to undergo adjuvant treatment (77.6% of patients with stage III vs. 48.3% of stage I patients, p<0.001), as were patients who underwent LAR (63.5%, p=0.010). Gender, race, marital status, patient comorbidity, tumor grade, and SEER region were not associated with a significant difference in adjuvant therapy initiation rates on univariate analysis.
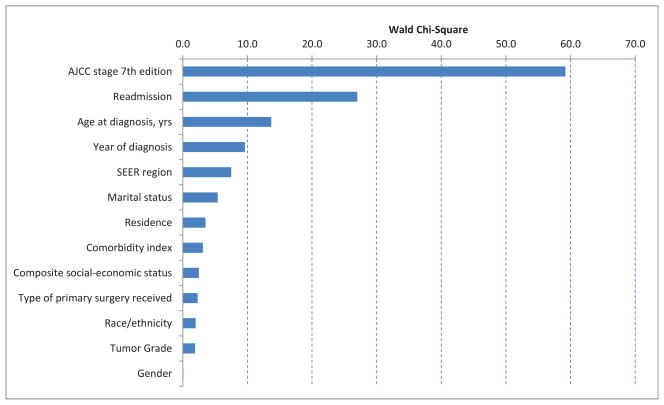
Figure 1 illustrates the effect size by hypothesis tests for each of the individual variables in the model. The horizontal bar (Wald Chi-square statistic) represents the strength of association with the outcome. Post-treatment pathologic stage of the tumor was strongly associated with chemotherapy initiation; patients with pathologic stage III tumors had an odds ratio (OR) of 3.87 (95% confidence interval (CI) 2.72, 5.51) for initiation of adjuvant therapy as compared to those with stage I tumors (Table 3). Patients with stage II or unknown post-treatment staging had intermediate odds (OR 1.57, 95% CI 1.16, 2.14 and OR 1.56, 95% CI 1.11, 2.18 respectively). Postoperative readmission was associated with decreased odds of initiating adjuvant therapy (OR 0.4, 95% CI 0.28, 0.56), as was increasing age at diagnosis (OR 0.58, 95% CI 0.43, 0.78 for ages 75–79). A more recent year of diagnosis was also associated with higher odds of initiation of adjuvant therapy (OR 1.64, 95% CI 1.06, 2.56 for years 2006–2007).
Figure 1.
Type 3 analysis of effect, derived from multivariate logistic regression.
Table 3.
Factors associated with initiation of adjuvant chemotherapy after rectal resection with preoperative chemoradiation and with the use of Oxaliplatin-containing regimen among those with chemotherapy initiated*
| Association with chemotherapy initiation | Association with the use of Oxaliplatin-containing regimen** | |||
|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Post-therapy pathologic stage | ||||
| I | 1 | Ref | 1 | Ref |
| II | 1.57 | 1.16–2.14 | 1.27 | 0.78–2.05 |
| III | 3.87 | 2.72–5.51 | 1.78 | 1.10–2.88 |
| Unknown | 1.56 | 1.11–2.18 | 0.94 | 0.54–1.61 |
| Postoperative readmission | ||||
| No | 1 | Ref | 1 | Ref |
| Yes | 0.40 | 0.28–0.56 | 1.11 | 0.68–1.81 |
| Age at diagnosis | ||||
| 65–69 | 1 | Ref | 1 | Ref |
| 70–74 | 0.67 | 0.52–0.88 | 0.5 | 0.35–0.70 |
| 75–79 | 0.58 | 0.43–0.78 | 0.57 | 0.38–0.84 |
| Year of diagnosis | ||||
| 1998–1999 | 1 | Ref | - | - |
| 2000–2001 | 0.95 | 0.61–1.48 | - | - |
| 2002–2003 | 1.16 | 0.75–1.79 | - | - |
| 2004–2005 | 1.31 | 0.85–2.02 | - | - |
| 2006–2007 | 1.64 | 1.06–2.56 | - | - |
model also adjusted for SEER region, marital status, residence, comorbidity index, SES, type of primary surgery received, race, tumor grade and gender
unadjusted for year of diagnosis as the approval of Oxaliplatin was in November 2004
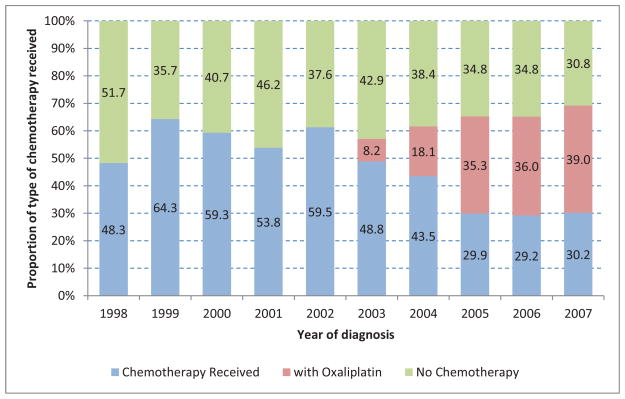
Temporal changes in both the proportion of patients undergoing adjuvant chemotherapy, and type of agents were noted. No patients received oxaliplatin prior to 2002. Beginning in 2003, an increasing proportion of patients received combination therapy. (Figure 2) In 2004 (year of FDA approval of oxaliplatin for colorectal cancer), only 18.1% of patients who underwent adjuvant therapy received an oxaliplatin-containing regimen. In 2007, this proportion increased to 39%. As shown by multivariate analysis on Table 3, older patients were less likely to receive a regimen including oxaliplatin (OR 0.57, 95%CI 0.38, 0.84 for patients aged 75–79) while patients with pathologic stage III tumors were more likely to have oxaliplatin included in their regimen (OR 1.78, 95%CI 1.10, 2.88).
Figure 2.
Type of chemotherapy received over time (N=1,492)
DISCUSSION
Current guidelines for the treatment of stage II and III rectal adenocarcinoma recommend neoadjuvant chemoradiation, followed by surgical resection and adjuvant chemotherapy. In this study of patients from the SEER-Medicare linked database, a large number of patients who underwent neoadjuvant therapy and subsequent resection did not receive adjuvant chemotherapy. This is unlikely to be explained by issues of access, especially as all patients in this cohort have been treated with preoperative chemoradiation and thus can be assumed to be connected with a medical oncologist. Additionally, treatment with neoadjuvant therapy is a marker for commitment to a multimodality strategy of care and coordination of teams to provide this care. Instead, patient and provider preferences and beliefs are likely to play a large role in decisions regarding adjuvant therapy in this setting.
The strongest predictor of initiation of adjuvant chemotherapy in this patient cohort was pathologic tumor stage; patients with post-treatment pathologic (yp) stage III tumors were nearly four times as likely as those with stage ypI tumors to receive adjuvant therapy. However, there are not good data to support using high post-treatment stage to determine need for further chemotherapy. In fact, some data suggest that those patients with no or limited response to 5-FU based neoadjuvant therapy (i.e. those with high pathologic stage) benefit least from adjuvant 5-FU chemotherapy while those with the better response (e.g. pathologic complete or partial response) benefit more.22 The data presented here suggest that current practice patterns do not reflect this and that further study is necessary to understand which patients gain a meaningful survival advantage from adjuvant therapy after neoadjuvant treatment and resection. While it is acknowledged that additional data regarding the benefits of adjuvant chemotherapy among patients with rectal cancer is needed, given the existing data, effort should be made to educate patients and health care providers on criteria for pursuing adjuvant therapy following rectal resection.
Surgical complications can lead to delay in initiation of adjuvant chemotherapy. However, prolonged postoperative recovery cannot fully explain gaps in adjuvant therapy administration. While patients in the SEER-Medicare cohort were less likely to receive adjuvant therapy if they were readmitted, nearly half still did, while under 2/3 of those without readmission initiated postoperative chemotherapy. These findings are consistent with previous studies which have demonstrated that complications are a significant factor in omission of postoperative chemotherapy, but only explain a minority of such omission.23 (Merkow et al, J Clin Oncol 30, 2012 (Suppl 4; abstr 551)) Inclusion of readmission in our model did not substantially affect the strong association between post-treatment stage and administration of adjuvant chemotherapy.
Age was also associated with the use of adjuvant therapy. Patients in the oldest group in this cohort (75 to 79 years old) had less than two-thirds the odds of the youngest (66 to 69) of initiating postoperative chemotherapy. This finding did not seem to be measurably mediated by comorbidity, since all patients in this cohort were deemed fit enough to withstand chemoradiation followed by surgery and non-cancer comorbidity (e.g. Charlson comorbidity score) was not a significant predictor in multivariate analysis. It is not possible to know the reasons for the decreased rate of adjuvant therapy use in older patients in this cohort, but this is similar to findings from a recent study of patients treated within the NCCN.8 A prior study of patients from the California Cancer Registry also found a strong association between advanced age and decreased use of chemotherapy, a relationship that held even for older patients without significant comorbidities.12 These and our results are in contrast to data from randomized trials in rectal cancer which have reported rates of adjuvant chemotherapy completion of 79–94%.24, 25 Thus this variation in use by age is likely reflective of both patient preferences and provider biases. Careful examination of these practices is necessary, especially as available data suggest that fit, elderly patients receive a similar advantage from adjuvant therapy as other groups.26–30
There are several notable temporal trends. The rate of initiation of adjuvant therapy following a neoadjuvant approach to rectal cancer has increased significantly from 1998 to 2007. This suggests that adherence to treatment guidelines is improving. Although there still remains a large group of patients who do not receive adjuvant chemotherapy, the 62% overall rate of adjuvant chemotherapy initiation is similar to the 42–75% adjuvant chemotherapy administration rate seen for elderly stage III colon cancer patients in clinical practice, although considerably lower than the rates achieved in randomized study.31 It is notable, however, that the population of rectal cancer patients in this study is biased towards a treatment-eligible cohort based on receipt of neoadjuvant chemoradiation therapy. Additionally, the rate of use of oxaliplatin-containing regimens has steadily increased since approval of this agent in 2004. However, these data also demonstrate that single-agent fluorouracil-based chemotherapy remains a common treatment choice for elderly patients undergoing rectal resection. Indeed there may be a group of patients who may not derive significant added benefit with the addition of oxaliplatin; however, there insufficient data at this time for routine chemotherapy treatment stratification.
There are several limitations to this study. As a retrospective database study, it is difficult to ascertain the precise reasons for omission of adjuvant therapy for individuals. Patients may defer further treatment after surgery, especially if their postoperative recovery is prolonged, however this issue would not be expected to disproportionately affect patients with early yp stage and inclusion of readmission in the model did not diminish the strength of the association with chemotherapy use. Difficulty with preoperative therapy may also influence decisions about postoperative treatment. However, while patient preference may contribute to this decision, the data here suggest that other factors may contribute substantially, especially postoperative stage. While the impulse to omit chemotherapy based on a good treatment response is on surface understandable, data does not exist to support this approach. In fact, data from the EORTC 22921 randomized trial suggests that those patients with a good response to neoadjuvant therapy (i.e. lower pathologic stage) derive the greatest benefit from adjuvant chemotherapy.22 It may be that the lack of response to 5-FU during concurrent chemoradiotherapy indicates resistance to 5-FU in the adjuvant setting and need for combination chemotherapy. Thus the rational selection of the postoperative regimen (i.e. decision to include oxaliplatin) may be possible based on the response to neoadjuvant therapy, although additional study is necessary to determine this. However, whatever the rationale for individual treatment decisions, our analysis suggests that this is already occurring, as patients with pathologic stage III tumors were more likely to receive oxaliplatin as part of their postoperative therapy. Another limitation of this data set is the lack of coding to consistently identify pathologic complete responders; these patients may have been classified as unstaged (Tx Nx). However, this is likely to represent fewer than 15% of patients following neoadjuvant chemoradiotherapy and any error introduced by these issues is unlikely to systematically influence the findings. Moreover, patients with metastatic disease were excluded and all patients in this cohort were given preoperative chemoradiation and are thus likely to be clinically stage II or III.
Our findings suggest that there is substantial variability in administration of adjuvant chemotherapy following neoadjuvant chemoradiation and resection for rectal cancer. While the precise etiology of this variability is not clear, it is notable that the patterns of care do not reflect the best available evidence; however they also serve to highlight the need for better evidence. Further study is necessary to illuminate the reasons for the observed deviation from guideline-based therapy in approximately 1 in 3 patients undergoing a neoadjuvant approach to adenocarcinoma of the rectum, let alone in those patients who do not undergo neoadjuvant treatment and are outside the scope of this study. Investigation of patient and provider decision-making will assist in ensuring that truly informed decisions are being made and that patients are receiving the full benefit of a multi-modality approach to rectal cancer therapy.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflicts of Interest:
None.
Disclosure:
Supported in part by a National Cancer Institute K07-CA133187 grant (G.J.C.).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D, Gelfand SE, Bach PB, Guillem J, Minsky BD, Begg CB. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from surveillance, epidemiology, and end results--Medicare. Journal of clinical oncology. 2001;19(17):3712–8. doi: 10.1200/JCO.2001.19.17.3712. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New England Journal of Medicine. 2004;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. Journal of clinical oncology. 2012;30(15):1770–6. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 7.Jacob S, Ng W, Asghari R, Delaney GP, Barton MB. Chemotherapy in rectal cancer: variation in utilization and development of an evidence-based benchmark rate of optimal chemotherapy utilization. Clinical Colorectal Cancer. 2011;10(2):102–7. doi: 10.1016/j.clcc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. Journal of clinical oncology. 2013;31(1):30–8. doi: 10.1200/JCO.2011.40.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110(3):660–9. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 10.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(8):1950–62. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. Journal of clinical oncology. 2002;20(5):1192–202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. Journal of clinical oncology. 2003;21(7):1293–300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Shah RV, Erkman LG, Hutchins LF. Racial differences in the outcome of patients with colorectal carcinoma. Cancer. 2003;97(2):493–8. doi: 10.1002/cncr.11067. [DOI] [PubMed] [Google Scholar]
- 14.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2006. [Google Scholar]
- 15.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–48. [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Gray R, Barnwell J, et al. Quasar Collaborative G. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 18.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 19.Park ES, Rabinovsky R, Carey M, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9(2):257–67. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 81–90. [DOI] [PubMed] [Google Scholar]
- 22.Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3–4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. Journal of clinical oncology. 2007;25(28):4379–86. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]
- 23.Merkow R, Bentrem D, Chow W, Cohen M, Ko C, Bilimoria K. Effect of postoperative complications on adjuvant chemotherapy use in stage III colon cancer. Journal of clinical oncology : Gastrointestinal Cancers Symposium. 2012;30(Supplement 4):551. [Google Scholar]
- 24.Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859–65. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 25.Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–87. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 26.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 27.Wildes TM, Kallogjeri D, Powers B, et al. The Benefit of Adjuvant Chemotherapy in Elderly Patients with Stage III Colorectal Cancer is Independent of Age and Comorbidity. J Geriatr Oncol. 2010;1(2):48–56. doi: 10.1016/j.jgo.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(25):4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 29.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294(21):2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 30.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–7. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 31.Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(21):2624–34. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]