Abstract
Radiation therapy after lymph node dissection increases the risk of developing painful and incurable lymphedema in breast cancer patients. Lymphedema occurs when lymphatic vessels become unable to maintain proper fluid balance. The sensitivity of lymphatic endothelial cells (LECs) to ionizing radiation has not been reported to date. Here, the radiosensitivity of LECs in vitro has been determined using clonogenic survival assays. The ability of various growth factors to alter LEC radiosensitivity was also examined. Vascular endothelial growth factor (VEGF)-C enhanced radiosensitivity when LECs were treated prior to radiation. VEGF-C-treated LECs exhibited higher levels of entry into the cell cycle at the time of radiation, with a greater number of cells in the S and G2/M phases. These LECs showed higher levels of H2A.X—an indicator of DNA damage—after radiation. VEGF-C did not increase cell death as a result of radiation. Instead, it increased the relative number of quiescent LECs. These data suggest that abundant VEGF-C or lymphangiogenesis may predispose patients to radiation-induced lymphedema by impairing lymphatic vessel repair through induction of LEC quiescence.
Keywords: lymphedema, lymphangiogenesis, radiosensitivity, lymphatic endothelium, VEGF-C
INTRODUCTION
Secondary lymphedema is a frequent and often dreaded side-effect of breast cancer treatment. The incidence of lymphedema is strongly correlated with axillary lymph node dissection followed by axillary radiotherapy [1]. Numerous studies have highlighted radiotherapy as an independent risk factor for lymphedema [2, 3]. Lymphedema occurs when lymphatic vessels become incapable of maintaining local fluid balance either through deficiencies in fluid uptake or the transport of lymph through larger collecting lymphatic vessels. Lymphedema is incurable and progressive, leaving patients with treatment options that are limited to palliative care. These treatments provide little relief, emphasizing the urgent need to develop methods that prevent or reverse the formation of lymphedema.
One potential treatment approach that has captured some interest in this field would employ lymphangiogenic growth factors to regrow the damaged vessels. The use of lymphangiogenic growth factors such as VEGF-C has been shown to reduce mild lymphedema in an animal model [4]. Unfortunately, strategies to induce lymphatic growth to correct the lymphatic deficits carry the risk of causing greater cancer dissemination if any cancer remains after therapy [5].
It is well documented that proliferating cells are more susceptible to ionizing radiation, so it is expected that the proliferating lymphatic endothelial cells (LECs) of lymphangiogenic vessels would be more radiosensitive than their quiescent counterparts. Lymphangiogenesis involving LEC proliferation can be triggered by post-surgical wound repair, which precedes radiotherapy. However, known lymphangiogenic growth factors can also provide survival signals to LECs leading to the alternative hypothesis that LEC survival and recovery may be enhanced by lymphangiogenic signaling during wound healing [6–8]. There are limited data describing the responses of LECs and lymphatic vessels to ionizing radiation, with the data showing heterogeneous radiosensitivity depending on the experimental conditions (i.e. radiation dose used and the lymphatic tissue bed of interest) [9–13]. These studies use radiation doses significantly higher than what a LEC would receive in a single radiation fraction clinically. This somewhat confounds these data, since the mechanisms that drive the endothelial cell response to radiation heavily depend on the single fraction radiation dose [14].
This work focuses on characterizing the response of dermal lymphatic endothelial cells to radiation. Here, standard colony formation assays were used to measure LEC radiosensitivity and compare it to blood vascular endothelial cells (BECs). Additionally, growth factors known to stimulate lymphangiogenesis—vascular endothelial growth factor (VEGF)-A[15], VEGF-C[16, 17] and basic fibroblast growth factor (bFGF)[18]—were examined for their abilities to alter radiosensitivity when administered to LECs before and/or after radiation.
MATERIALS AND METHODS
Cell models
Human adult dermal lymphatic endothelial cells (LECs) and human adult dermal blood vascular endothelial cells (BECs) were obtained from Lonza (Basel, Switzerland) and grown in EGM™-2MV Microvascular Endothelial Cell Medium-2 (Lonza) and used between passage 3 and 8. The starvation media (SF) consisted of the basal EBM media with the addition of hydrocortisone, gentamicin/amphotericinB, and ascorbic acid. The full growth media (GM) consisted of the starvation media supplemented with VEGF-A, basic fibroblast growth factor (bFGF), R3-insulin-like growth factor-1, epidermal growth factor and 5% fetal bovine serum. The media additives to the EBM media that were used for the SF and full GM were purchased as part of the Lonza EGM ™2MV Bulletkit™ and used as directed. Cells were cultured on plastic or glass that was pre-coated with 0.001% fibronectin (Sigma) dissolved in PBS.
Cell radiation and feeder cells
A broad-field 250 kVp x-ray irradiator with a dose rate of 1.89 Gy/min (Siemens Stabliplan 2; Siemens AG, Munich, Germany) was used for all radiation experiments. To maintain constant seeding density, lethally radiated BECs or LECs were plated as feeder cells with their respective viable cells so that each well had a total (viable plus feeder) of 20000 cells plated. Feeder cells were generated by administering 20 Gy in one fraction.
Clonogenic survival assay
Clonogenic survival assays were performed on BECs and LECs to determine their baseline radiosensitivities. These assays were based on the previously described clonogenic survival assay protocol [19]. Briefly, LECs and BECs were cultured in full growth media and plated onto 6 well-plates pre-coated with 0.001% fibronectin. Based on the plating efficiency, two different cell densities were plated for 0, 1, 2 and 3 Gy doses (1,600 and 3,200 LECs for the 0 Gy dose, 3,200 and 6,400 LECs for 1 Gy, 6,400 and 12,800 LECs for 2 Gy, 9,600 and 19,200 LECs for 3 Gy). 20,000 viable LECs were seeded in all wells for 4, 5, 6 and 8 Gy as lower seeding densities yielded insufficient colony formation. After incubating overnight in GM, LECs were incubated in SF for 48 h prior to radiation to induce cell quiescence (Figure 1A). After irradiation, cells were immediately washed and replaced with SF for additional 48 h. Cells were then placed in GM for two weeks, with media changes every 2 to 3 days. After 14 days of incubation, cells were stained with crystal violet. Colonies of greater than 50 cells were counted and the survival fraction was calculated using:
| (Eqn.1) |
with the plating efficiency for each group calculated by its corresponding 0 Gy dose using:
| (Eqn. 2) |
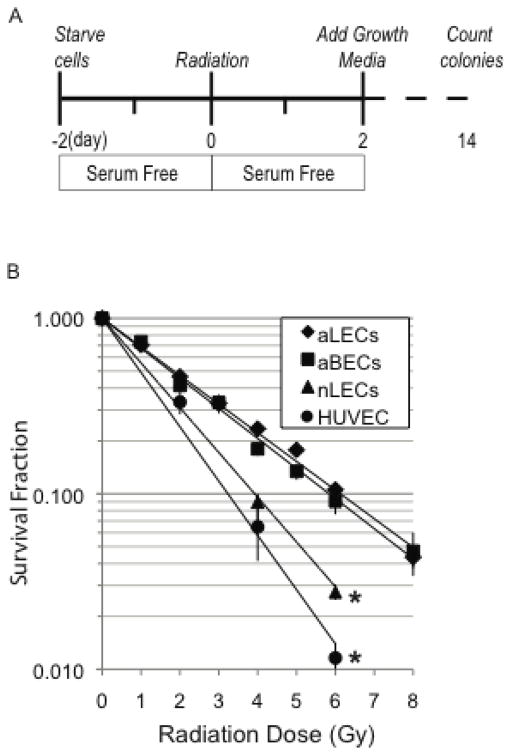
Figure 1. Radiosensitivity of adult LECs and adult BECs is similar.
Adult and neonatal lymphatic endothelial cells (aLEC and nLEC) were compared to adult blood endothelial cells (aBEC) and human umbilical vein endothelial cells (HUVEC) using clonogenic survival assays. Curves for aLECs and aBECs show no difference in dose-responsive survival fraction between the two. nLECs and are more radiosensitive than their adult counterparts as measured by their reduced survival fractions. * p<0.05 comparing nLECs vs. aLECs and aBECs vs. HUVECs. Statistical comparisons were made using a general linear model as described in the methods.
Radiosensitivity of LECs with growth factors
The approach for these assays is summarized in Figure 2A. To determine whether selected lymphatic growth factors (VEGF-C, VEGF-A, bFGF) alter the radiosensitivity of LECs, growth factors (GFs) were added to LECs 48 h prior to irradiation and removed immediately after irradiation for the duration of the clonogenic survival assay (Pre-treatment Assay). Conversely, to determine whether lymphatic GFs alter the ability of LECs to repair DNA damage and survive radiation-induced damage, GFs were added for 48 h after LEC irradiation (Post-treatment Assay).
Figure 2. VEGF-C reduces the survival fraction of LECs.
Growth factors were tested using four experimental arms in clonogenic survival assays (a). Dose-responsive radiosensitivity of aLECs in response to VEGF (b), bFGF (c) and VEGF-C (d) were measured. *, p<0.05 comparing SF/SF vs VEGF-C/SF (d). Statistical comparisons were made using a general linear model as described in the methods.
LECs were plated as described above. After incubating overnight in GM, LECs were divided into four groups. The first group (SF/SF) was incubated in SF for 48 h prior to irradiation. After irradiation, cells were immediately washed and replaced with SF for additional 48 h. The second group (SF/GF) was also incubated in SF for 48 h prior to irradiation. After irradiation, cells were immediately washed and replaced with SF supplemented with a GF of interest (human VEGF-A (R&D Systems; 50 ng/ml), human VEGF-C (R&D Systems; 500 ng/ml) or human bFGF (R&D Systems; 10 ng/ml)). These concentrations were chosen based on previously published determinations of effective dosages in LECs and BECs [20]. The third group (GF/GF) was incubated in SF supplemented with a GF of interest for 48 h prior to irradiation. After irradiation, cells were immediately washed and replaced with SF supplemented with a GF of interest. The fourth group (GF/SF) was incubated in SF supplemented with a GF of interest for 48 h prior to irradiation. After irradiation, cells were immediately washed and replaced with SF for an additional 48 h. Cells were then placed in GM for two weeks, with media changes every 2 to 3 days. After 14 days of incubation, cells were stained with crystal violet. Colonies of greater than 50 cells were counted and the survival fraction was calculated using Equation 1.
Statistical analysis
To compare curves of clonogenic survival versus time under different experimental conditions a General Linear Model was used of the form:
| (Eqn.3) |
Where Survival Fraction was calculated using Eqn. 1, Dose was the amount of radiation in Gy, Group described the 4 groups (SF/SF, SF/GF, GF/GF, SF/GF), and Experiment denoted the independent repetitions of the assay (n=3). The variables A+B+C denoted the doses for each experimental group (SF/GF, GF/GF, SF/GF) to be compared to the control (SF/SF). This set-up was the equivalent of a Dose*Group variable. Significance of any of the variables A, B and C on ANOVA identified experimental groups that had a different slope of the dose response curve compared to the slope of the control dose response curve. Statistical significance was considered when p < 0.05. For all other assays, the standard t-test or ANOVA was used to determine statistical significance as appropriate.
Cell cycle analysis and apoptosis detection
For cell cycle analysis, approximately 1×106 LECs were fixed in 90% methanol for 4 h, followed by incubation with 25 ug/ml propidium iodide dissolved in PBS supplemented with 0.1% TritonX. Cell cycle analysis and quantitation were performed by flow cytometry using the BD LSRII and BD FACSDiva software. The average number of cells in each cell cycle phase was determined from 3 to 7 samples per experimental group. For detection of radiation-induced apoptosis, LECs were fixed in 3% formaldehyde followed by 90% methanol and immunostained with cleaved caspase-3 (CC3) antibody 5A1E (Cell Signaling) according to the manufacturer’s protocol. The total number of CC3-positive cells was quantified by flow cytometry from 3 to 5 replicates.
Immunofluorescence Microscopy
Cells were fixed in 4% formaldehyde in PBS and permeabilized in 0.3% TritonX in PBS. For detection of DNA damage, immunofluorescence was performed with anti- H2A.X-FITC antibody (Millipore). Cells were imaged by confocal microscopy using a 60X objective. The total number of positive foci per nucleus was determined manually. For determination of quiescence, immunofluorescence was performed with anti-human Ki67 antibody (ab15580, Abcam), and cells were imaged by confocal microscopy using a 20X objective. The total number of positive cells per field was determined manually. All microscopy was performed using an Olympus Bx61w1 confocal microscope.
RESULTS
The radiosensitivity of quiescent adult lymphatic endothelial cells (aLECs) was measured using colony formation assays and was shown to be similar to adult blood microvessel endothelial cells (aBECs) (Figure 1). The data were fit to a simple exponential model and D0 (the dose required to achieve 37% cell survival) was calculated as 2.6 Gy. The surviving fraction at 2 Gy (SF2) was 0.48. Interestingly, the neonatal LECs (nLECs; D0=1.6, SF2=0.34) and human umbilical vein endothelial cells (HUVECs; D0=1.3, SF2=0.27) were more radiosensitive than the adult counterparts (Fig. 1B). The radiosensitivity of adult LECs was similar to adult BECs, although our LEC plating efficiency of 6.4 1.0% was somewhat lower than our data and other published results for BECs (10–15%) [21–23].
LEC radiosensitivity in response to lymphangiogenic growth factors
In order to test the ability of a growth factor to act as a radiosensitizer or a radioprotector, four experimental arms were arranged for each clonogenic survival assay (Fig. 2A). The four arms tested whether the presence of the growth factor prior to radiation would have a different effect on LEC radiosensitivity compared to the presence of the growth factor after radiation. Neither bFGF nor VEGF altered the radiosensitivity of LECs when present before or after radiation (Fig. 2B, C). VEGF-C, however, reduced colony formation when present before radiation (GF/SF vs. SF/SF, p<0.05) (Fig. 2D). Three independent experiments showed similar, statistically significant responses (p<0.05), though there was variability in the magnitude of the response between each of the experiments (data not shown). The groups in which VEGF-C was added after radiation also showed a modest increase in radiosensitivity, though due to the intra-experimental variability, particularly at the higher radiation doses, statistical significance was not achieved (Fig. 2D). None of the growth factors tested showed any radioprotective effects towards LECs in these clonogenic assays.
Cell cycle profiles of irradiated LECs
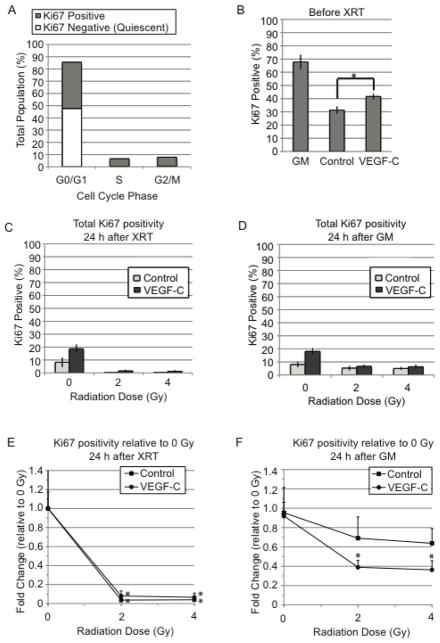
Radiation causes a delay in cell cycle progression in most eukaryotic cells. The result is a visible accumulation of cells in one or all phases of the cell cycle, depending on the cell type examined. Cell cycle analysis of LECs showed that the percentage of LECs in G0/G1 increased from 69.8+/−1.0 to 87.5+/−1.5 with a corresponding decrease in the percentage in S and G2/M when LECs were deprived of serum and growth factors (p<0.05, Table 1). When treated with VEGF-C, a higher percentage of LECs were in S and G2/M (9.6+/−1.4 and 10.6+/−0.4, respectively) compared with control (5.0 +/− 0.9 and 7.5 +/− 1.0, respectively) (p<0.05, Table 1). 24 h after radiation, while LECs were still in serum- and growth factor-free media (SF), all treatment groups maintained high G0/G1 fractions between 86.3% and 91.1% (no statistical difference between samples). When media on this group of cells was switched from SF to growth media (GM) for an additional 24 h, LECs from both control and VEGF-C groups that had received no radiation exhibited strong increases in S and G2/M populations with corresponding drops in G0/G1 (p<0.05 for all phases relative to status in SF media 24 h after radiation). LECs that had received either 2 Gy or 4 Gy 48 h earlier showed modest increases in S and G2/M in response to GM stimulation for 24 h. These groups maintained higher G0/G1 fractions than their 0 Gy counterparts (p<0.05, Table 1), indicating that addition of GM was limited in its ability to promote cell cycle progression of the radiated LECs in both control and VEGF-C treated groups.
Table 1.
Cell cycle analysis of LECs.
| Cell Status | Treatment Group | Radiation Dose (Gy) | Phase (% Total Cells +/− s.e.)
|
||
|---|---|---|---|---|---|
| G0/G1 | S | G2/M | |||
| Asynchronous (cells in GM) | n.a. | n.a. | 69.8 +/− 1.0 | 16.4 +/− 12 | 13.9 +/− 2.1 |
| At XRT (cells in SF) | Control | n.a. | 87.5 +/− 1.5 | 5.0 +/− 0.9 | 7.5 +/− 1.0 |
| VEGF-C | n.a. | 79.9 +/− 1.3 | 9.6 +/− 1.4 | 10.6 +/− 0.4 | |
| 24h after XRT (cells in SF) | Control | 0 | 90.6 +/− 2.3 | 4.6 +/− 1.4 | 4.8 +/− 1.3 |
| 2 | 91.1 +/− 2.2 | 4.7 +/− 2.0 | 4.3 +/− 1.5 | ||
| 4 | 91.1 +/− 1.4 | 3.4 +/− 0.9 | 5.6 +/− 1.6 | ||
| VEGF-C | 0 | 86.6 +/− 1.5 | 6.5 +/− 2.6 | 6.9 +/− 1.7 | |
| 2 | 87.4 +/− 0.9 | 5.1 +/− 2.3 | 7.5 +/− 2.0 | ||
| 4 | 86.3 +/− 2.0 | 5.1 +/− 3.0 | 8.3 +/− 2.7 | ||
| 48h after XRT SF to GM switch for the last 24h | Control | 0 | 65.4 +/− 2.2 | 18.9 +/− 2.3 | 15.6 +/− 2.1 |
| 2 | 84.6 +/− 2.9 | 8.2 +/− 2.0 | 7.1 +/− 2.2 | ||
| 4 | 86.1 +/− 2.7 | 6.4 +/− 1.6 | 7.5 +/− 1.8 | ||
| VEGF-C | 0 | 67.6 +/− 3.1 | 16.1 +/− 2.8 | 16.3 +/− 2.2 | |
| 2 | 79.8 +/− 3.8 | 8.8 +/− 1.4 | 11.3 +/− 2.9 | ||
| 4 | 82.4 +/− 3.4 | 6.9 +/− 1.8 | 10.6 +/− 2.3 | ||
s.e. standard error of the mean; XRT, radiation; Control, cells pre-incubated in starvation media; VEGF-C, cells preincubated in starvation media plus 0.5 ug/ml VEGF-C; SF, serum and growth factor-free media; GM, growth media; n.a., not applicable. The percentage of cells in the indicated cell cycle phase is shown.
VEGF-C treatment predisposes LECs to increased DNA Damage
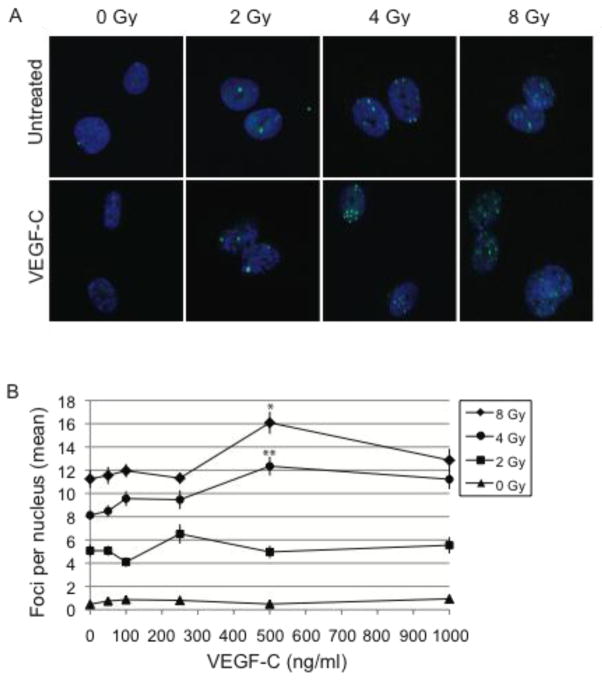
The higher S and G2/M levels of VEGF-C-treated LECs suggested that LECs may be sensitized to radiation because of their more proliferative phenotype at the time of radiation. To test this, H2A.X was used to compare the level of DNA damage in the control and VEGF-C-treated groups. H2A.X is a phosphorylated histone that localizes to sites of double strand DNA damage [24]. These sites are visible by immunofluorescence microscopy as sub-nuclear foci. The extent of DNA damage was determined by counting the number of foci per nucleus for 30–50 nuclei per condition (Fig. 3A). The average was then taken as a gross indicator of the level of DNA damage (Fig. 3B). As expected, the number of foci increased proportionally to the dosage of radiation. Additionally, the number of H2A.X foci increased with increasing concentrations of VEGF-C up to 500 ng/ml (p<0.05) at both 4 and 8 Gy of irradiation (Fig. 3B).
Figure 3. Pre-treatment with VEGF-C increases DNA damage.
Radiation-induced DNA damage in LECs was detected by immunofluorescence for H2A.x 20 h after radiation. Sub-nuclear foci formed by H2A.X were imaged using confocal microscopy, scale bar 10 μm. Images shown represent LECs treated with 500 ng/ml VEGF-C (a). Dose-responsive effects were examined by pre-treating LECs with 0, 50, 100, 250, 500 or 1000 ng/ml VEGF-C. The total number of foci per nucleus was counted and averaged. Data were analyzed by ANOVA with Tukey’s Honestly-Significant-Difference Test post-hoc for each radiation dose (b). blue, DAPI; green, H2A.X; *, p<0.05 when compared to 0 ng/ml, 50 ng/ml 100 ng/ml, 250 ng/ml and 1000 ng/ml of VEGF-C; ** p<0.05 when compared to 0 ng/ml, 50 ng/ml, 100 ng/ml and 250 ng/ml of VEGF-C.
VEGF-C pretreatment is protective against LEC apoptotic cell death
When cells are unable to repair damaged DNA, apoptosis and cell death often occur. To test whether VEGF-C-induced DNA damage in LECs resulted in an increase in apoptosis, LEC expression of the apoptotic marker cleaved caspase-3 (CC3) was quantified. As expected, the CC3 level remained low for LECs maintained in GM while increasing for both the control and VEGF-C groups, which had been deprived of serum and growth factors prior to radiation. Surprisingly, VEGF-C reduced CC3 levels in all groups at 6 hours after radiation (p<0.05 for all groups). While a radiation-induced, dose-responsive increase in CC3 was evident in the control group (p<0.05), the VEGF-C group did not show a similar responsiveness (Fig. 4). Various time-points ranging from 3 h to 48 h showed similar dynamics, with VEGF-C reducing the number of CC3-positive cells (data not shown). These data indicate that VEGF-C reduction in colony-formation is not due to increased apoptosis, as VEGF-C may have a protective effect against radiation-induced apoptosis.
Figure 4. VEGF-C does not enhance radiation-induced cell death.
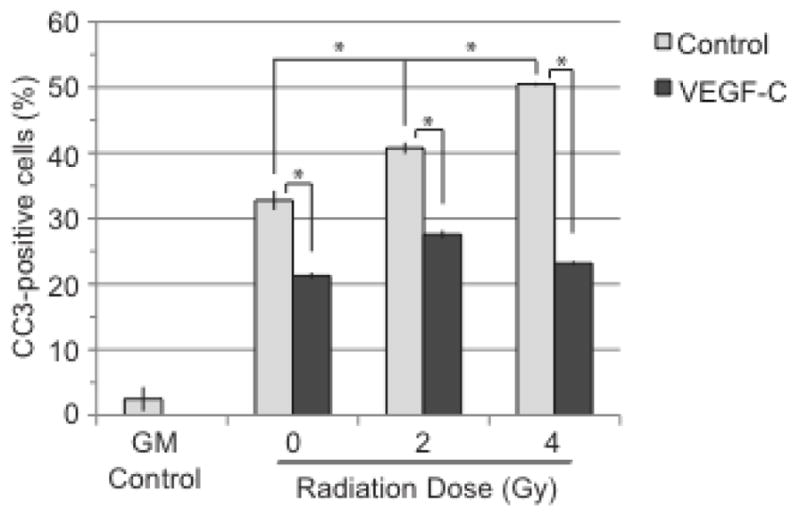
LECs were collected 6 h after radiation, stained for cleaved caspase-3 (CC3) and collected by flow cytometry. GM control is represented by LECs that have been grown in regular growth media. All other groups were starved prior to radiation. 0 Gy control groups did not receive radiation. Statistical analysis was performed using the standard t-test. *, p<0.05
VEGF-C pretreatment promotes radiation-induced quiescence
Since VEGF-C did not enhance apoptosis in response to radiation, a quiescent phenotype in LECs caused by VEGF-C-enhanced DNA damage was tested next. Under this model, the lack of colony-formation would be explained by the loss of LEC reproductive capacity through cell cycle arrest rather than by loss of LECs through cell death. Ki67 is a well-established marker for assessing cellular proliferation. It is a nuclear protein that is expressed by a cell when it has entered into the active phases of the cell cycle (G1, S, G2 and M) but is not expressed when the cell is quiescent (G0) [25]. Ki67 was used to distinguish the populations of cycling cells from quiescent cells (Fig. 5A). At the time of radiation, the VEGF-C-treated group showed higher Ki67 positivity than the control group (p<0.05), consistent with the well-documented proliferative role of VEGF-C on lymphatics as well as the cell cycle profiles presented here (Fig. 5B, Table 1). Ki67-positivity in all groups dropped 24 h after radiation, with radiated groups exhibiting >90% reductions relative to the 0 Gy controls (Fig. 5C, E). When growth media (GM) was added to stimulate proliferation, Ki67-positivity did not increase substantially in either control or VEGF-C groups after 0 Gy (Fig. 5D). However, irradiated groups exhibited increases in Ki67-positivity in response to GM (Fig. 5E, F), suggesting that GM can revive the proliferation potential of LECs after irradiation. VEGF-C treated LECs, however, did not recover to the level of the 0 Gy VEGF-C group (p<0.05, Fig. 5F).
Figure 5. Pre-treatment with VEGF-C increases LEC quiescence.
Flow cytometry for Ki67-positivity in high passage LECs (p8) showed Ki67 as a distinguishing marker between cycling and quiescent cells in the G0/G1 population (a). The percentage of Ki67 positive cells was determined visually using immunofluorescence microscopy. The control and VEGF-C groups were compared at the time of radiation (b), 24 h after radiation while under starvation (c), and 24 h after GM addition (d). Fold changes relative to 0 Gy control were determined for both groups at 24 h after radiation while under starvation (e) and 24 h after addition of GM (f). Statistical significance for 2 and 4 Gy groups was determined relative to 0 Gy (e,f). Statistical analysis was performed using the standard t-test. GM, growth media; XRT, radiation; *, p<0.05
DISCUSSION
Lymphedema remains an incurable side-effect of radiotherapy. The lack of preventative and targeted treatments is due largely to a scarcity of knowledge regarding the mechanisms that drive LEC vessel dysfunction. This study reports the first LEC radiosensitivity curves, demonstrating that LEC radiosensitivity is similar to BECs. The results of four independent experiments showed a LEC plating efficiency of 6.4 1.0%. The plating efficiency of various blood vascular endothelial cells has been reported to be between 10 and 15% [21–23], which is similar to the observations presented here with aBECs. The radiosensitivity of HUVECs in this study was also comparable to previously published data [6, 7, 26]. The reason for the lower plating efficiency of LECs relative to BECs is unclear.
As part of the experimental design, 48 hours of starvation was used to induce cell quiescence in order to mimic a stable, non-proliferative endothelium. It has been hypothesized that a quiescent endothelium is less sensitive. Initial experiments to determine the radiosensitivity of the blood vessel endothelium showed great variability that was also attributed to the proliferating nature of the endothelial cells in culture in comparison to the limited proliferation of endothelial cells in normal blood vessels in vivo [21, 26].
Further examination of LEC radiosensitivity in response to VEGF-C indicates that, like most cell types, LECs are more sensitive to radiation when they are more proliferative at the time of radiation. By prompting cell cycle entry with physiological levels of VEGF-C, more DNA-damage is apparent in response to radiation. Paradoxically, apoptosis as a result of radiation is reduced when VEGF-C is present, indicating that the pro-survival role of VEGF-C towards the endothelium remains intact. A reduction in the number of Ki67 positive cells, however, shows that the LECs are no longer proliferating. This observation is consistent with a finding that has documented radiation-induced senescence of LECs of the lymphatic vessels in the mouse tail [9]. Our observation of quiescent LECs is also consistent with the observation that lymphatic vessels remain functional and capable of handling tissue edema shortly after the initial radiation dose [11]. The data presented here indicate that the presence of VEGF-C before and during radiation is protective against radiation-induced cell death; however, VEGF-C-treated LECs are still severely damaged and incapable of further cell division. Thus pre-existing lymphatic vessels may survive irradiation and remain functional but may not be able to produce new lymphatic vessels in response to edema formation. These data also suggest that the presence of VEGF-C in tissue before, during and perhaps directly after radiation may predispose patients to lymphedema since LECs cannot proliferate sufficiently to repair damaged vessels.
After axillary node dissection, there is a healing wound bed that promotes proliferation of endothelial cells and growth of new blood and lymphatic vessels as part of the healing process. It is during this healing process—the period in which cells are most vulnerable—that radiation is often administered to sterilize any remaining residual disease. The question remains as to whether anti-lymphangiogenic therapy would be beneficial when administered prior to and concurrent with radiotherapy. We hypothesize that the presence of VEGF-C may help predict lymphedema in women after axillary radiotherapy for breast cancer metastasis. Furthermore, VEGF-C may be a target to prevent lymphedema when targeted prior to radiotherapy.
These data also suggest that adult endothelial cells are less radiosensitive than their neonatal counterparts, both for the blood and lymphatic endothelium. It will become important to determine at what age a child’s endothelial cells become less sensitive to radiation in order to better understand treatment effects in pediatric cancer patients.
These findings will need to be validated in vivo, where questions about the radiation dose and timing relative to surgery can be addressed, and the impact of VEGF-C on lymphatic radiosensitivity can be further explored. Moreover, the contributions of additional lymphangiogenic microenvironmental factors—including fibroblasts and macrophages—before, during and after radiation will need to be investigated. A fundamental understanding of these responses will guide radiation treatment planning, highlight therapeutic strategies to prevent radiation-induced lymphedema in cancer patients and potentially identify patients at greater risk for developing lymphedema.
Acknowledgments
This work was funded by NIH R00CA137167, NIH DP2OD008780 and NCI Federal Share/MGH Proton Beam Income on C06 CA059267. We would like to thank Dr. Rakesh Jain, Dr. Brian Seed, Dr. Dai Fukumura, Dr. Jay Loeffler, Nicole Magpayo and members of the Edwin L. Steele Laboratories for helpful discussions. We would also like to thank Dr. David Schoenfeld and Harvard Catalyst Biostatistical Consulting.
Footnotes
Conflict of Interest Notification: There are no conflicts of interest related to the work presented in this manuscript.
References
- 1.Warren AG, et al. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59(4):464–72. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 2.Meek AG. Breast radiotherapy and lymphedema. Cancer. 1998;83(12 Suppl American):2788–97. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2788::aid-cncr27>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Hinrichs CS, et al. Lymphedema secondary to postmastectomy radiation: incidence and risk factors. Ann Surg Oncol. 2004;11(6):573–80. doi: 10.1245/ASO.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Tammela T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13(12):1458–66. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 5.Gershenwald JE, I, Fidler J. Targeting lymphatic metastasis. Science. 2002;296(5574):1811–2. doi: 10.1126/science.10731318. [DOI] [PubMed] [Google Scholar]
- 6.Gorski DH, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–8. [PubMed] [Google Scholar]
- 7.Gupta VK, et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8(1):47–54. doi: 10.1097/00130404-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Karkkainen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 9.Avraham T, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol. 2010;299(3):C589–605. doi: 10.1152/ajpcell.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackowski S, et al. Radiogenic lymphangiogenesis in the skin. Am J Pathol. 2007;171(1):338–48. doi: 10.2353/ajpath.2007.060589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon AM, et al. The effects of radiation on the contractile activity of guinea pig mesenteric lymphatics. Lymphology. 1994;27(4):193–200. [PubMed] [Google Scholar]
- 12.Mortimer PS, et al. Time-related changes in lymphatic clearance in pig skin after a single dose of 18 Gy of X rays. Br J Radiol. 1991;64(768):1140–6. doi: 10.1259/0007-1285-64-768-1140. [DOI] [PubMed] [Google Scholar]
- 13.Sung HK, et al. Intestinal and peri-tumoral lymphatic endothelial cells are resistant to radiation-induced apoptosis. Biochem Biophys Res Commun. 2006;345(2):545–51. doi: 10.1016/j.bbrc.2006.04.121. [DOI] [PubMed] [Google Scholar]
- 14.Ch’ang HJ, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11(5):484–90. doi: 10.1038/nm1237. [DOI] [PubMed] [Google Scholar]
- 15.Nagy JA, et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–37. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 16.Joukov V, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(7):1751. [PMC free article] [PubMed] [Google Scholar]
- 17.Kukk E, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122(12):3829–37. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 18.Kubo H, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99(13):8868–73. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franken NA, et al. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 20.Makinen T, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee JG, Lee I, Song CW. The clonogenic response of bovine aortic endothelial cells in culture to radiation. Radiat Res. 1986;106(2):182–9. [PubMed] [Google Scholar]
- 22.Cho MM, et al. Estrogen modulates paracellular permeability of human endothelial cells by eNOS- and iNOS-related mechanisms. Am J Physiol. 1999;276(2 Pt 1):C337–49. doi: 10.1152/ajpcell.1999.276.2.C337. [DOI] [PubMed] [Google Scholar]
- 23.Abdollahi A, et al. SU5416 and SU6668 attenuate the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Res. 2003;63(13):3755–63. [PubMed] [Google Scholar]
- 24.Paull TT, et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10(15):886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 25.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Donker M, et al. Negligible radiation protection of endothelial cells by vascular endothelial growth factor. Oncol Rep. 2007;18(3):709–14. [PubMed] [Google Scholar]