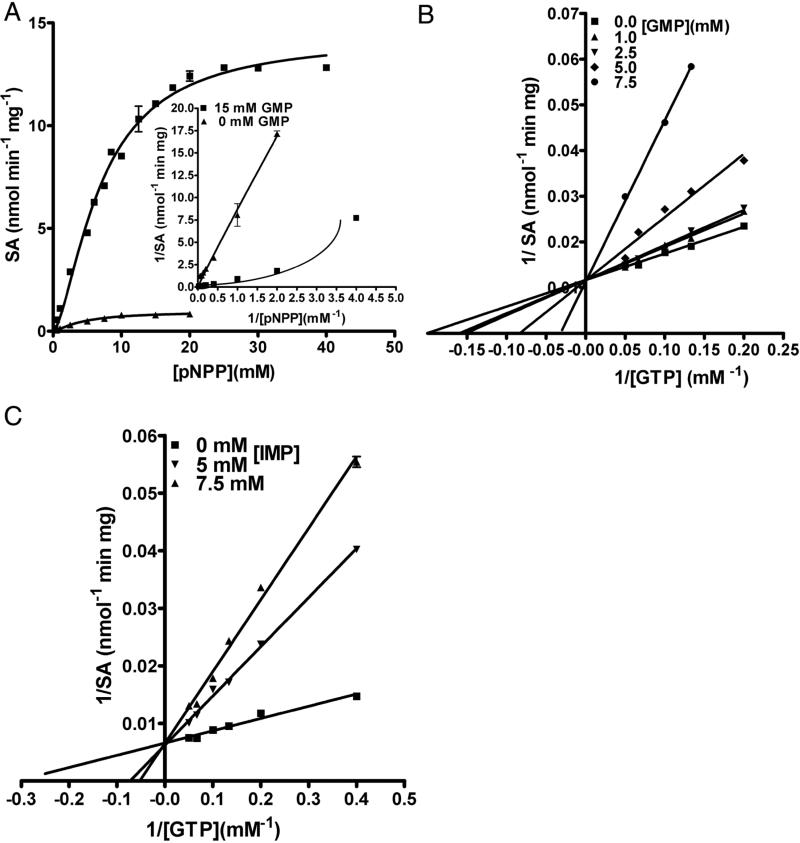
Figure 3.
Activation of pNPP hydrolysis by GMP. (A) pNPP titration was carried out at 0 mM and 15 mM GMP concentration and monitored for pNP formation at 405 nm. The inset shows the double reciprocal plot for pNPP titration in the presence and absence of GMP. Note the non-linearity of the curve in the presence of GMP, characteristic of enzymes exhibiting sigmoidal behavior. pNPP titration, in the absence of GMP, was stopped at 20 mM because pNPP exhibits substrate inhibition. (B) Reduction in GTP activation of pNPP hydrolysis by GMP. The liberation of pNP was monitored at 405 nm. Each line corresponds to the titration of [GTP-Mg2+] at various fixed concentration of GMP. (C) Reduction in GTP activation of pNPP hydrolysis by IMP. The liberation of pNP was monitored at 405 nm. Each line corresponds to the titration of [GTP-Mg2+] at various fixed [IMP]. Data were fitted by linear regression and non-linear curve fitting algorithms of GraphPad prism Software, version 4.0 (GraphPad Software, Inc., San Diego, CA).