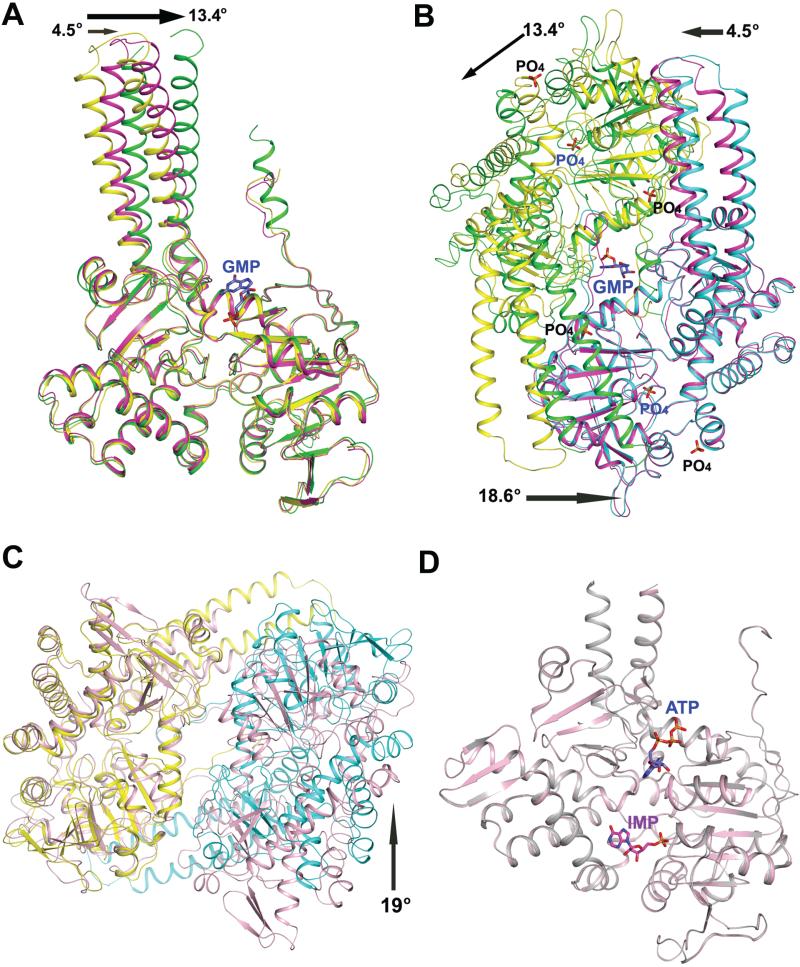
Figure 7.
Structural changes upon activator binding in human cN-II and LpcN-II. (A) Superposition of the PO4-complexed subunit A (yellow) and subunit A (green) and subunit B (magenta) of the GMP-complexed LpcN-II structures. The rotation (in °) of the helical extension in each subunit of the GMP-complexed structure relative to that of the PO4-complexed structure is shown by an arrow. (B) Superposition of the PO4-complexed subunit B (cyan) and the GMP-complexed subunit B (magenta) of LpcN-II, showing the activator binding site and the rearrangement of subunit A (green) in the GMP-complexed structure relative to the PO4-complexed structure (yellow). The activator binds in a groove at the dimer interface. The rotation (ino) of the helical extension in each subunit and the core of the GMP-complexed structure relative to the position of each subunit of the PO4-complexed structure is shown by an arrow. (C) Superposition of the human apo dimer structure (pink, PDB ID 2XCX) and LpcN-II PO4-complexed (yellow/cyan) dimer structure (PDB ID 4G63). Only subunit A from each structure (yellow in PO4-complexedstructure ) was included in the structural alignment. The arrow indicates a rotation (in o) of the subunit B of the PO4-complexed LpcN-II structure with respect to human apo structure. (D) Structural alignment of human cN-II apo form (in pink, PDB ID 2J2C) and in complex with IMP and ATP (in gray, PDB ID 2XCW).