Figure 1.
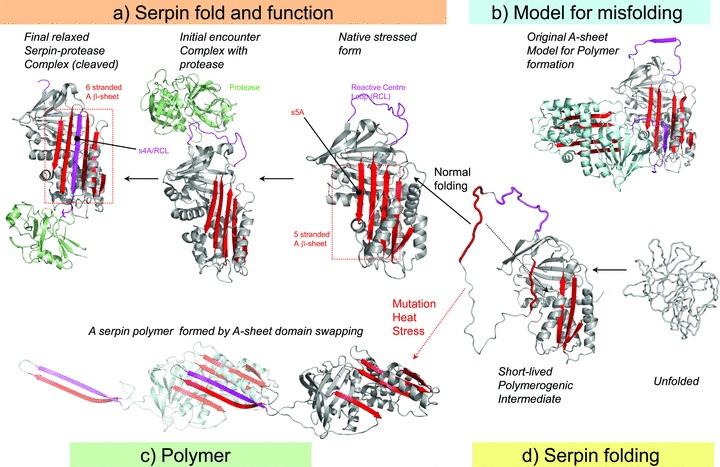
Two models for polymerization of ATZ. (A) Active serpins fold into a metastable state. Following the initial interaction with a target peptidase and reactive center loop (RCL) cleavage, the serpin undergoes a radical conformational change (RCL/s4A incorporation into b‐sheet A) that culminates in peptidase inhibition via distortion of the catalytic residues. (B) Loop‐sheet model of serpin polymerization. The RCL of one molecule is inserted into the open b‐sheet A of another. (C) Domain swapping model of serpin polymerization. This model is based on the structure of a domain‐swapped antithrombin dimer, where s5A and s4A (RCL) of a donor molecule insert into b‐sheet A of a recipient. (D) Normal serpins may fold through a polymerogenic intermediate that is stabilized by certain mutations. The black dotted arrow indicates a gap in b‐sheet A that accommodates s5A to form the s4A (RCL) exposed native molecule to A or the s5A and s4A domain‐swapped structure in C. Reproduced with permission from Figure 2 of Whisstock JC et al. J Biol Chem. 2010; 285: 24307–24312.
